Epileptic Disorders
MENUThe effect of oral administration of monosodium glutamate on epileptogenesis in infant rats Volume 22, numéro 2, April 2020
Monosodium glutamate (MSG), which is used for the flavouring of foods, was first isolated by Kikunae Ikeda in the early 1900s from dried konbu and was used in traditional Japanese soup (Ikeda, 2002). MSG was also used in Far East cuisine and has also been commonly used in Western countries over the years (Bellisle et al., 1991). MSG is an L-glutamate (L-GLU) derivative, which is soluble in water. When specific tongue receptors are stimulated, MSG is tasted as an umami taste, the fifth basic taste. The receptors that mediate the effects of MSG on the gastrointestinal system arefound in the stomach (Kondoh and Torii, 2008).
GLU, a non-essential amino acid, is the main excitatory neurotransmitter of the central nervous system (CNS) (Schousboe, 1981). At high concentrations, it is distributed in the cerebral cortex, cerebellum, and hippocampus in the brain. In addition to its physiological importance, it plays a key role in the pathophysiology of temporal lobe epilepsy (TLE) (Engel et al., 1997). TLE is the most common type of partial epilepsy in humans and in some cases, insufficient drug treatment causes a serious health problem (Eid et al., 2004).
In some studies, parenteral or oral administration of MSG has led to contradictory results. Parenteral administration of MSG has been shown to cause damage, especially in the hypothalamus, as well as increased short stature, metabolic derangement, and weight gain (Diniz et al., 2004; Fernandez-Tresguerres Hernández, 2005; Matyskova et al., 2008). Low concentrations of orally administered MSG have been shown to increase saturation, regulate appetite, and decrease sodium consumption without changing taste (Bellisle, 2008; Masic and Yeomans, 2014). However, high concentrations of MSG given orally caused metabolic disorders, depending on nutrition (Diniz et al., 2005). In another study, it was indicated that both oral and parental administration led to voracious behaviour (Hermanussen et al., 2006).
In previous studies, it was demonstrated that MSG caused tonic and clonic convulsions after high-dose and parenteral administration in adult rats, and reduced the convulsion threshold in newborn rats when taken orally in late pregnancy (Yu et al., 1997; González-Burgos et al., 2004). In a recent study, it was observed that epileptiform activity, induced by 4-aminopyridine, significantly increased in rats exposed to parental administration of MSG in the neonatal period (Hernandez-Ojeda et al., 2017).
In this study, we added MSG to the drinking water of rats that were just weaned and had not completed their development with an aim to investigate its role in the onset and spread of temporal lobe seizures via the hippocampal kindling model, as well as effects on weight gain and nasal-anal length (NAL).
Materials and methods
Animals and experimental design
Twenty-four Wistar Albino rats (six females and six males in each group, age 21 days, 75-95 g) supplied from Marmara University Animal Center (DEHAMER) were used in the study. All experimental procedures were approved by the local ethics committee, MUHDEK (Approval No. 46.2013.mar). The rats were housed with a reversed 12-hour light/dark cycle at 21 ± 3̊C and 50 ± 5% humidity. There was unlimited access to standard rat chow.
The infant rats were weighed and divided into two groups, the MSG group and control group, and 1.0 g/L MSG (Sigma-1446600) and 1.0 g/L NaCl were added to the drinking water of the rats in the MSG group and control group, respectively. In order to prevent possible low consumption and mask the bad taste, 24 g/L sucrose was added to the water of both groups and the rats were provided with 24-hour unlimited access to this water. The weight of the animals was recorded from the beginning to the end of the experiment. On the 30th day of the experiment, their first NAL measurements were taken and stereotaxic surgery was conducted under anaesthesia. According to the group, water with MSG or NaCl was continued, and the animals were allowed to recover for one week.
On the 37th day of the experiment, when the animals were aged two months, the kindling procedures were started and continued until the rats were kindled. At the end of the experiment, the last NAL and weight measurements were taken under high-dose sodium thiopental anaesthesia. The animals were decapitated and their brains were removed for histological analysis. Only the results of rats with confirmation of the targeted brain region were used.
Kindling
On the 30th day of the experiment, standard stereotaxic surgery was conducted on the animals under 75-mg/kg ketamine and 10-mg/kg xylazine (i.p) anaesthesia. Two stainless steel screws with attached insulated wire were implanted into the left frontal and left parietal cortex for EEG recording. An electrical stimulation electrode (MS303/1 twisted, Plastic's One Inc., Roanoke, VA, USA) was implanted into the right hippocampus (AP -3.8 mm; ML -2.2 mm; DV-2.3 mm from the bregma; Paxinos and Watson, 1998). After a one-week recovery period, on the 37th day of the experiment, basal EEG records were obtained for one hour and electrical stimulation threshold values were determined. Animals with a threshold of over 500 μA were removed from the experiment. Rectangular constant current pulses were applied to the animals twice a day for 2 ms at their determined threshold values. EEG was recorded for one hour in total, 30 minutes before and after each stimulation. Electrical stimulations were applied according to Racine's scale (RS) to reach Stage 3 and display three consecutive Stage 5 seizures (Racine, 1972). Those thatreached Stage 5 up to the 25th stimulus were considered kindled. EEG records were evaluated using a BioAmp ML 136 amplifier and Chart v7 program (PowerLab8S ADI Instruments, Oxfordshire, UK).
Data analysis
All data are expressed as mean ± standard error of the mean (SEM). GraphPadPrism 5.03 software was used for the analysis of the data. The groups were compared using the unpaired two-tailed t (normally distributed) and Mann Whitney U (non-normally distributed) tests. For all statistical calculations, significance was considered at p<0.05.
Results
Evaluation of weight
The weight of the animals was recorded throughout the experiment and their weight on the first day and 37th day (the first day of the kindling process) of the experiment were compared. Although the weight of the male rats in both MSG and control groups was not significantly different (p>0.05), the weight of female rats in the MSG group was significantly increased compared with the control group (table 1, figure 1).
Evaluation of nasal-anal length (NAL)
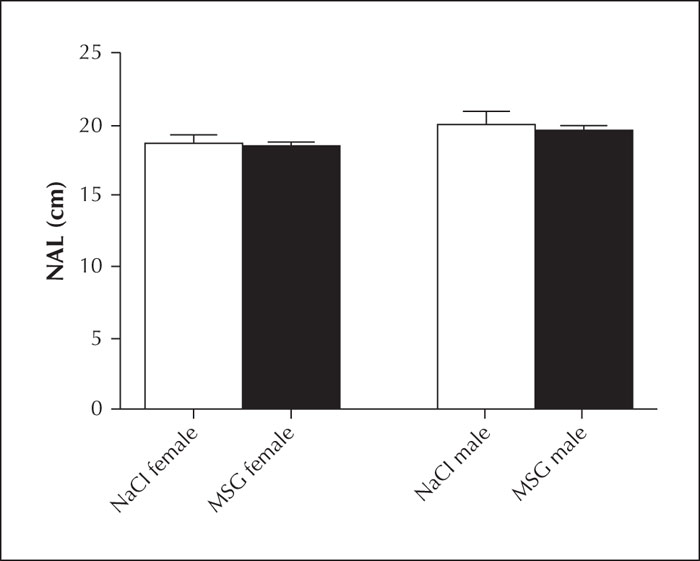
NALs measured just before the kindling process were compared between males and females in both groups. No significant difference was found between the NALs of male and female rats in the MSG and control group (table 2, figure 2).
The effect of oral MSG administration on the kindling process
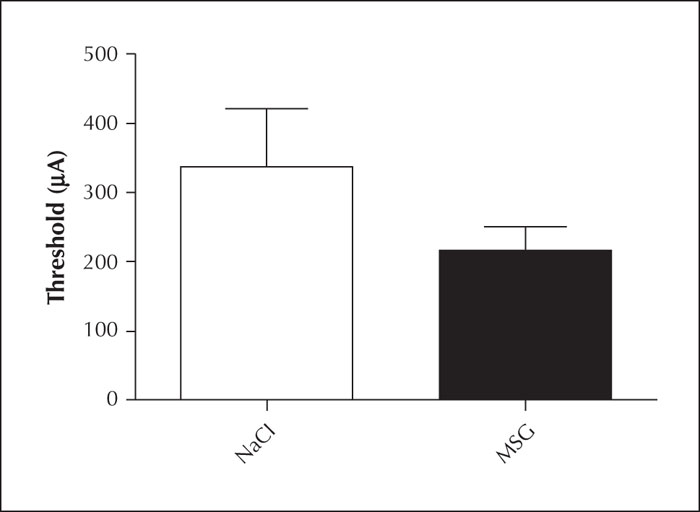
No significant differences were found in the electrical stimulation threshold values, determined on the first day of the kindling process, between the MSG group and the control group (table 3, figure 3).
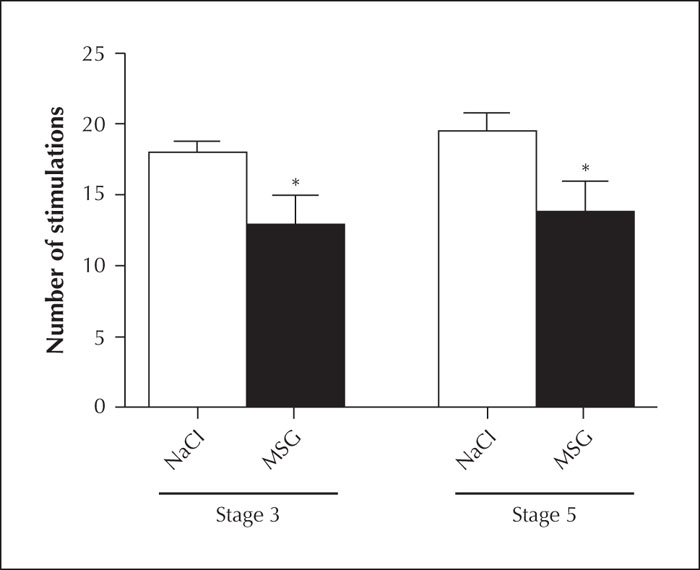
A statistically significant difference was found in the number of stimulations required to reach Stage 3 and Stage 5 based on the RS in the MSG group compared with the control group (p<0.05) (table 4, figure 4).
Discussion
In this study, we investigated the effects of orally administered MSG on height and weight as well as on the CNS. Although oral administration of MSG did not affect the height of either sex, it increased weight gain of the female rats (figure 1).
Previous studies have demonstrated that only orally administered MSG was effective through binding to specific receptors in the gastrointestinal tract. These receptors are taste-mGluR4 (Chaudhari et al., 2000) and T1R family receptors, which create a heterodimeric structure (Li et al., 2002), located in taste buds on the tongue. Although MSG produces umami taste with specific tongue receptors, it stimulates the vagus nerve, probably via increased production of bioactive substances such as nitric oxide and serotonin by activating the specific stomach receptors (Kondoh and Torii, 2008). In light of this knowledge, exogenous GLU, probably via afferent vagus nerve fibres, stimulates the lateral hypothalamic area (LHA), which is part of the autonomous nervous system and plays a critical role in the regulation of eating and drinking behaviour, in addition to the insular cortex and basal ganglia, and increases weight gain in animals. Thus, in the event of total or partial vagotomy, food intake and weight gain decreases (Kondoh et al., 2000). In one study, a decrease in synaptic plasticity (long-term potentiation) in the hippocampal CA1 region occurred in rats fed a high-fatdiet for an extended period, which was associated with weight gain in the animals (Hwang et al., 2010). In other words, a decrease in hippocampal synaptic plasticity may be a facilitative cause of weight gain by affecting the hypothalamus, which is more sensitive to these effects. However, the fact that weight gain occurred only in female rats in our experiment makes us consider that another mechanism may also play a role in weight gain. Oestrogen and GLU have a bidirectional relationship. Whereas oestrogen reduces blood and brain GLU concentrations, GLU stimulates the production of oestrogen and increases its blood concentrations (Zlotnik et al., 2011). In our study, oral consumption of GLU may have induced weight gain in female rats by making metabolic changes through oestrogen.
Another, and perhaps more important, finding of our study was that the MSG group reached Stage 3 based on the RS faster than the control group although equivalent stimulation thresholds were applied to the animals (figure 4). Stage 3 is an indicator that epileptic activity covered the brain hemisphere where stimulation was applied and is a significant stage in epileptogenesis (Racine, 1972). An increase in GLU concentration, which is the main excitatory neurotransmitter of the CNS, causes oxidative stress while overstimulation of its receptors causes neuronal apoptosis and necrosis mediated by increasing intracellular Ca2+ (Patel et al., 1996). Neuronal damage may cause neurodegenerative diseases in addition to the emergence of epileptic seizures (Gill et al., 2000). However, it is commonly held that the MSG concentration added to foods does not affect brain GLU concentrations (Fernstrom, 2018). Our study reveals a significant finding in this regard: even oral consumption of GLU salts speeds up the emergence of epileptogenesis and facilitates animals to be kindled (figure 4).
Kindled or Stage 5 according to the RS demonstrates that the stimulation does not stay in the hemisphere where it is applied, but spreads to the entire brain leading to generalized seizures (Racine, 1972). Although it would seem unlikely that GLU, taken at low concentrations over a short period, could cross the blood-brain barrier and become more concentrated in the brain, a high dose and/or repeated exposure of GLU may have caused neurotoxicity upon crossing the barrier. Oral administration of MSG has been shown to lead to behavioural changes that could be expressed as anxiogenic behaviour (Narayanan et al., 2010). Moreover, some studies have demonstrated that exogenous MSG could cause damage in the brain and organs such as the kidney and liver by creating oxidative stress or/and an excitotoxic effect (Eweka and Om’Iniabohs, 2008). These effects, emerging after oral administration, may be due to the fact that MSG can cross the blood-brain barrier when administered at high concentrations over extended periods or can cause oxidative stress via receptors in the periphery. Additionally, we can consider that this effect of exposure to MSG at an early stage of life may be because the CNS is more sensitive and the blood-brain barrier is more permeable, as development is not yet complete (Fernandez-Tresguerres Hernández, 2005). Orally administered GLU speeds up epileptogenesis and facilitates kindling that may arise from an affected hippocampus which plays a critical role in epilepsy, similar to vagus nerve afferent fibres affecting the LHA, leading to weight gain. As another hypothesis, orally administered GLU may have caused an increase in sensitivity to GLU in the hypothalamus as a result of changes in the periphery, creating oxidative stress.
In previous studies, it was observed that parenterally administered MSG facilitates the kindling process in various types of models (Castellanos et al., 1998; Mori et al., 1989). This is due to glutamate being an excitatory amino acid. Moreover, the preferred routes of administration in these models cannot be used in humans. In our study, unlike other studies, we used a model whereby MSG is administered as a food additive. We started to give MSG to rats orally on the day they were just weaned (21stday), equivalent to an approximately two-year-old human. In terms of CNS development, the end of the second month in rats corresponds to the age of 12 in humans.
In conclusion, although orally administered GLU salts are known to be well metabolized and only a small amount enterssystemic circulation, its extended use may cause the emergence of hyperexcitability in the brain and facilitate neurodegenerative disorders, particularly epilepsy. Therefore, it would not seem appropriate to consume such food additives during a period when the development of the blood-brain barrier and CNS is not yet complete.
Disclosures
None of the authors have any conflict of interest to declare.