Epileptic Disorders
MENUMultimodal assessment of language and memory reorganization: a proof of concept in two patients with drug-resistant temporal lobe epilepsy Volume 21, numéro 5, October 2019
Temporal lobe epilepsy (TLE) accounts for 70-80% of epilepsy cases in adults (Jaimes-Bautista et al., 2015). The underlying dysfunction (epileptogenic zone [EZ]), which is located in the temporal lobe, induces language and memory reorganization of the cerebral networks that are associated with varying degrees of cognitive efficiency (see Roger et al. [2018] for a review). Around 35% of TLE patients are refractory to antiepileptic drugs (i.e. drug-resistant), and curative neurosurgery remains, in this case, the only therapy capable of stopping seizures (Harroud et al., 2012). Given the reorganization of language and memory functioning related to recurrent seizures, the mapping of functional networks is an essential step in the pre-surgical phase (Hamberger, 2007).
fMRI studies have shown that TLE patients exhibit atypical language representations at either inter-hemispheric or intra-hemispheric level, mainly resulting from: (a) inter-hemispheric displacement accompanied by involvement of the non-dominant hemisphere; (b) cross-lateralized distribution with a partial shift of eloquent regions to the right hemisphere; and (c) intra-hemispheric reorganization of networks extending beyond the classic eloquent regions of the dominant hemisphere, accompanied by the recruitment of non-traditional language regions (see Baciu and Perrone-Bertolotti [2015] for a more detailed review of these patterns). The functional reorganization of the memory processes in TLE is more difficult to assess owing to its widely distributed representation in the brain (Mosca et al., 2014). Bilateral temporal regions (medial and lateral), among others, are involved in the encoding and retrieval memory processes (Eliassen et al., 2008, 2012). Some variations on the functional activations of these regions, depending on the modality involved (verbal, non-verbal) for instance,complicate the reorganization assessment at the hemispheric level alone. Moreover, some regions thought to be essential in the memory functioning are specifically impacted in TLE. TLE is very often accompanied by hippocampal sclerosis, and given the key role of this medial region in episodic encoding and recollection (Simons and Spiers, 2003), it may be expected that memory is impaired in these patients (Binder et al., 2008). However, the fact that some hippocampal sclerosis patients do not exhibit memory deficits suggests that in TLE patients, memory may depend on other regions and on complex reorganization patterns.
Our understanding of epilepsy has significantly increased in recent years (Kramer and Cash, 2012; Stam and van Straaten, 2012; Engel, 2013; Jiruska and De Curtis, 2014) due to the development of structural connectivity methods (Diehl et al., 2008; Braakman et al., 2012; Dinkelacker et al., 2016). Magnetic resonance diffusion tensor imaging (DTI) in epileptic patients compared to non-epileptic subjects has revealed diffuse white-matter (WM) changes in multiple bundles, including the commissural fibers and those located at a distance from the EZ (Arfanakis et al., 2002; Concha et al., 2005, 2010; van Eijsden et al., 2011). Duffau and colleagues (Duffau et al., 2014) have demonstrated, using WM direct electrical stimulation, the crucial part played by subcortical pathways in the neural circuitry involved in cognition. The authors underscored the core role of subcortical connectivity in shaping cortical reorganization following perturbations of cognitive functions, and focal epilepsy - classically described as a cortical disease - has emerged as a pathology of networks (Kramer and Cash, 2012; Stam and van Straaten, 2012; Engel, 2013).
Patients with TLE present disorganization of both language and memory bundles (for a review, see McDonald et al. [2008]; Rodríguez-Cruces and Concha, 2015), as language and memory networks converge towards integrative hubs that mainly stem from the left temporal lobe (Mesulam, 2000; Holland and Lambon Ralph, 2010; Battaglia et al., 2011; Guo et al., 2013). Several studies have evaluated the correlation between various anatomical, functional and cognitive parameters to describe modifications occurring in TLE patients. For example, Kucukboyaci and colleagues investigated the relationships between DTI and volumetric MRI on one hand and performance deficits in a naming task in TLE patients on the other. The authors suggest that neither cortical thickness nor grey or white matter contrast in fronto-temporal regions fully account for behavioural performance. On the contrary, WM diffusion parameters were strongly correlated with performance, even after controlling for hippocampal volume (Kucukboyaci et al., 2014). In the same vein, James and collaborators (James et al., 2015) observed significant correlations between functional lateralization indices for language representation, as assessed with fMRI, and structural connectivity asymmetry indices calculated using parameters derived from DTI. Thus, to obtain a complete neurocognitive picture, a multimodal approach based on multiple datasets reflecting brain activation, cerebral structure, and cognitive performance appears essential.In addition, given the importance of the WM integrity in cerebral functioning, a complete cortical and subcortical mapping in TLE patients could be routinely used to guide decisions about neurosurgery (e.g. the extent of the cortical resection), as well as to reduce the risk of language and/or memory impairment following the resection.
In the present study, we applied this multimodal approach to report two cases of TLE patients who underwent DTI, fMRI, and neuropsychological assessments. The data from these assessments were interpreted in an integrative fashion and were compared to those obtained from a group of healthy individuals. Our aim was to describe the relationships between WM bundle microstructure, brain activity for a language-memory task, and cognitive performance for language and memory in particular. DTI allowed to measure fractional anisotropy (FA) extracted from the main intra- and inter-hemispheric WM bundles involved in language and memory. Functional activity for language was assessed via a naming task that is part of a standard fMRI protocol at the University Hospital Centre of Grenoble (NEREC protocol) (Perrone-Bertolotti et al., 2015). Lateralization indices on frontal activations were then computed to gain insight into hemispheric specialization for language. DTI and fMRI data for patients were interpreted based on cognitive scores for language and memory, with a view to measuring the efficiency of reorganization patterns. The two cases we present here are of interest for two main reasons: (1) they underscore the importance of factoring in inter-subject variability and more informative descriptions of neurocognitive functioning, regardless of the similarity of the EZ locations; (2) both cases emphasize the importance of taking into account structural connectivity in order to generate comprehensive neurocognitive profiles.
Material and methods
Participants
Control group
Twelve healthy volunteers (mean age: 23.92; SD: 2.02; nine females) were included in the study. All participants were native French speakers, had normal or corrected-to-normal vision, were right-handed (Edinburgh Handedness Inventory [Oldfield, 1971]), and reported no neurological or psychiatric disorders. Participants (controls and two patients) provided written informed consent to participate in the study, which was approved by the local ethics committee (CPP: 09-CHUG-14/ANSM [ID RCB] 2009-A00632-55).
Patients
Two patients with the same presumed localization of the epileptogenic zone (drug-resistant mesial temporal lobe epilepsy) were included in the study. They also matched in structural lesions (hippocampal sclerosis [HS] and temporo-polar blurring phenomenon), age at onset/frequency of seizures as well as for the number of daily-taken antiepileptic drugs. There were, however, differences between the two patients, in particular, regarding manual laterality and duration of the disease. The details of the clinical features presented by these patients are shown in table 1.
Experimental evaluation
Both Patient 1 and 2 underwent neuropsychological, DTI, and fMRI examinations, whereas the control group underwent fMRI and DTI examinations only. For both groups, functional MRI and DTI were performed using a whole-body 3T MR scanner (Philips Achieva) with 40 mT/m gradient strength. The different acquisition parameters and the neuropsychological tests used are shown in table 2.
Regarding the fMRI paradigm, we applied the NEREC protocol (Naming Encoding RECognition (Perrone-Bertolotti et al., 2015), which is a two-in-one inter-mixed language-and-memory protocol. We specifically used the naming task (based on the DO80 database, a French equivalent of the Boston Naming Test) because of its ability to map basic language skills needed on everyday life (lexical retrieval and generation). For a more complete description of the task and its design paradigm, refer to Perrone-Bertolotti et al. [2015].
Data processing
Neuropsychological scores
We mainly focused on the scores obtained for language and memory tasks. Each score was standardized via a statistical comparison with the normative sample (matched by age, gender, and education) specific to each of the validated neuropsychological tests that we used in this study. Standardized scores were indicated in percentile rank (ct) or in standard deviation to the norm (σ). In agreement with clinical practice and a tolerance threshold at p ≤ 0.05, a normative score equal to or less than 5ct or -1.65σ was considered as pathological.
DTI data analysis
The DICOM raw data for each DTI acquisition was converted to the NIFTI format using the dcm2niix toolbox v1.0.20170429, before being concatenated and pre-processed via the Diffusionist software tool (Boisgontier et al., 2009; Delouche et al., 2016), running on Linux and based on FSL v5.0 (FMRIB Software Library v5.0, Oxford UK) (Smith et al., 2004; Woolrich et al., 2009) and DTK v0.6.4 (Diffusion ToolKit, Wang and Wedeen, Trackvis.org, Martinos Centre for Biomedical Imaging, Massachusetts General Hospital) scripts. In a first step, the concatenated data was corrected (Eddy current correction; realignment). The brain extraction for each subject was then performed (Bet brain extraction: binary mask of the brain without scalp). Diffusion tensors were estimated using the DTK method. The tensors were spectrally decomposed in order to obtain the eigenvalues (λ1, λ2, λ3) and eigenvectors (e1, e2, e3). Fractional anisotropy (FA) maps of all participants (patients and controls) were then extracted and standardized on an MNI (Montreal Neurological Institute) template using the non-linear image registration tool FNIRT (Non-linear Registration Tool, Andersson, Jenkinson, Smith, 2010). Our aim was to assess possible changes in WM at (a) the intra-hemispheric level by measuring the FA of the following fascicules of interest (FOIs): inferior longitudinal (ILF), inferior fronto-occipital (IFOF), superior longitudinal (SLF), arcuate (Arc), uncinate (Unc), cingulum (Cing) and fornix (Fox); and (b) the inter-hemispheric level, by considering the WM fibres associated with the following sub-regions of the corpus callosum (CC): genu (GCC), body (BCC) and splenium (SCC). The FOIs were delineated using the JHU ICBM-DTI-81 (Mori et al., 2005) and Catani (Catani and Thiebaut de Schotten, 2008) atlases implemented in Diffusionist. The mean FA values from these FOIs were then extracted for the patients and the control group. For each FOI, we compared the mean FA values of each of our two patients (independently) with the control group, based on modified Crawford t test (t’), using Singlims software (Crawford and Howell, 1998; Crawford and Garthwaite, 2002). The threshold was set at p < 0.05. The t’ values and associated probability were indicated. A complete table of the mean FA values obtained for Patient 1 and 2 and healthy subjects is available in the additional material section (supplementary table 1).
Functional MRI data analysis
SPM12(www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB 2015b (Mathworks Inc., Natick, MA, USA) was used to analyse fMRI data. Following pre-processing of the functional MR images, the volumes were time-corrected using the 22nd slice as a reference to correct artefacts that had occurred during the time elapsed between each instance of slice acquisition. All of the volumes were then realigned to correct for head motion, using a rigid body transformation. T1-weighted anatomical volume was co-registered to mean images created by the realignment procedure and was normalized within the MNI space. Anatomical normalization parameters were used for the normalization of functional volumes. Each functional volume was smoothed using a Gaussian kernel of 8 mm FWHM (full width at half maximum). A time series for each voxel was then high-pass filtered (1/128-Hz cut-off) to remove low-frequency noise and signal drift. Statistical analyses were then performed using the pre-processed data - initially at an individual level. For each participant, we modelled two regressors of interest (task/naming and control/rest) as a box-car function convolved with a canonical hemodynamic response. Movement parameters derived from the previously mentioned realignment correction were entered in the design matrix as six additional regressors of no interest. Subsequently, for the control group, a statistical analysis at a group level (n=12) was performed, using a one-sample t-test (k > 5; p<0.05 FWE corrected; t = 6.5) to assess activation for the main group-related contrast (task vs. control). Activated regions were identified and labelled via a macroscopic parcellation of the MNI single subject reference brain (Tzourio-Mazoyer et al., 2017).A table of the activation peaks obtained during the naming task by Patient 1 and 2 and healthy subjects is available in the additional material section (supplementary table 2).
Based on fMRI activation of the frontal region, we generated frontal lateralization indices (LI) using the LI-tool (Wilke and Lidzba, 2007; Alary et al., 2013) implemented in the SPM12 toolbox. As previously noted, this tool calculates typical lateralization indices based on left and right hemisphere activation (Baciu et al., 2005; Seghier, 2008). LI calculation was based on the number of activated voxels at a regional level, given that this method seems to be slightly less vulnerable to statistical outliers (Wilke and Lidzba, 2007). We focused the LI calculation on the frontal regions owing to the very high level of congruence with the Wada lateralization test, which is traditionally used to indicate global hemispheric specialization for language (Lehéricy et al., 2000). For these purposes we used a mask based on symmetrical left-right anatomical frontal regions (Tzourio-Mazoyer et al., 2017). We then calculated frontal LIs for each patient and for the control group.
Results
Patient 1
fMRI results for hemispheric specialization for language
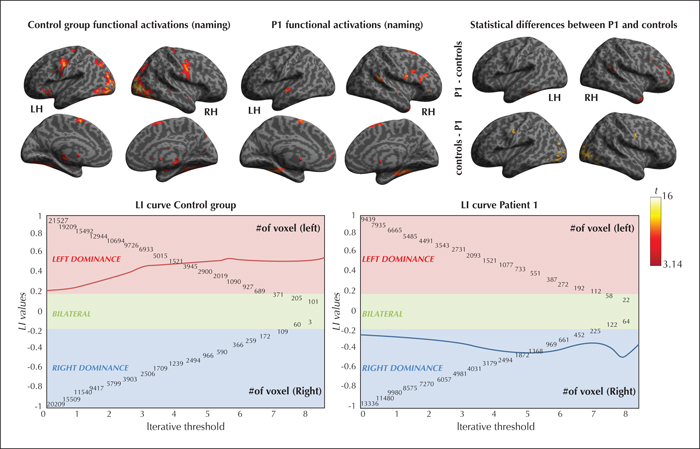
At p <0.05corrected (t ≥ 6.5 and k > 5) threshold, Patient 1 frontal LI was -0.412, suggesting a right hemisphere specialization for naming; whereas the controls were left-hemisphere lateralized (see figure 1 for the functional activation maps and supplementary table 1 in the supplementary material section; Seghier [2008]).
DTI results
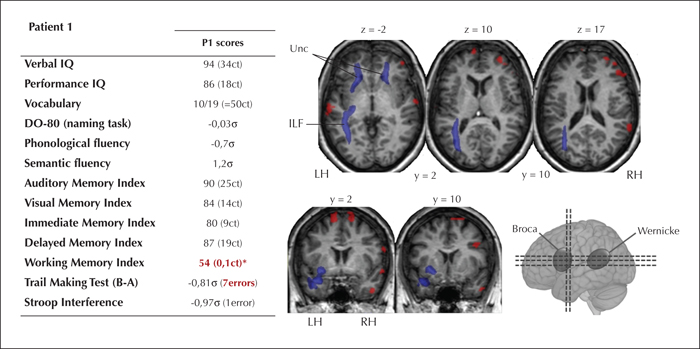
At p ≤ 0.05, the FA of a series of WM bundles was significantly lower in Patient 1 relative to the controls (see figure 2 and supplementary table 2 in the supplementary material section). On the left side, the altered intra-hemispheric fascicles were the ILF (t’(12) = -2.169, p = .03), the Unc (t’(12) = -1.778, p < 0.05), and the Fox (t’(12) = -1.801, p < 0.05); and on the right, the Unc (t’(12) = -1.922, p = 0.04). No inter-hemispherical differences were observed in the corpus callosum (t’(12) = -0.18, p = 0.4).
Cognitive scores
Patient 1 showed normal performance for verbal and memory tests. However, when faced with heightened demands in executive skills for memory tasks (working memory essentially), Patient 1's performance was deficient (WM index < 1ct). The deficits of Patient 1 mainly occurred in the realm of executive functioning (figure 2).
The multimodal results for this patient are summarised in figure 2.
Patient 2
fMRI results for hemispheric specialization for language
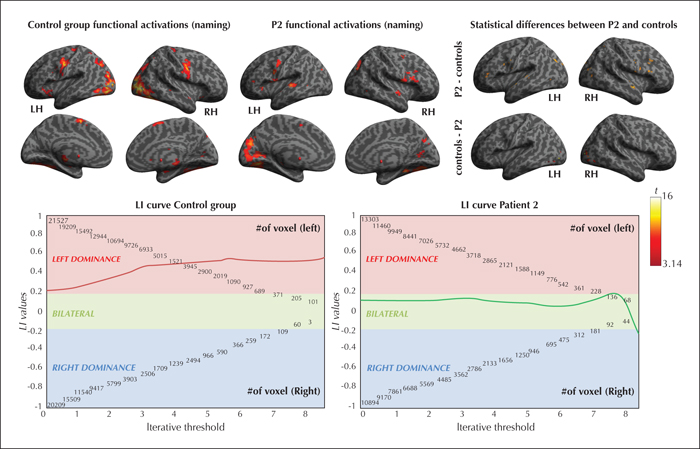
At p <0.05corrected (t ≥ 6.5 and k > 5), frontal LI in Patient 2 was 0.165 (compared to the controls who were left-hemisphere lateralized), suggesting a bilateral frontal representation of language for Patient 2 (see figure 3 for the functional activation maps and supplementary table 2 in the supplementary material section; Binder et al. [1996], Seghier [2008]).
DTI results
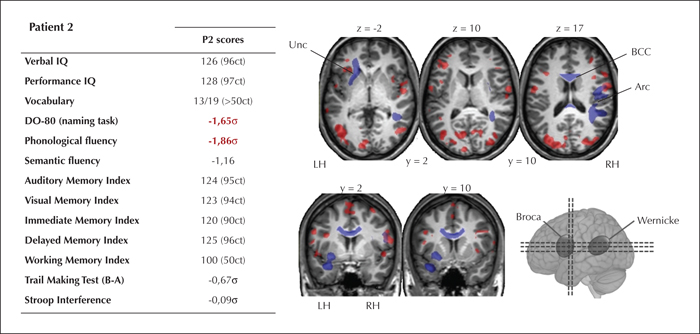
Multiple WM bundles (see figure 4 and supplementary table 2 in the supplementary material section) exhibited significantly lower FA values (p ≤ 0.05) for Patient 2, compared to the control group. Patient 2 had significantly lower FA for the: left Unc (t’(12) = -1.671, p < 0.05); right Arc (t’(12) = -3.431, p < 0.05); and for the BCC (t ’(12) = -1.922, p <0.05), compared to the controls.
Cognitive scores
As can be seen in figure 4, Patient 2 had normal results for memory tests for both verbal and non-verbal material. As for language, despite Patient 2's normal scores for verbal comprehension and vocabulary, the scores were pathological for tests involving phonological and lexico-semantic processes (phonological fluency = -1,86 σ; object naming = -1,65 σ).
The multimodal results obtained for this patient are summarised in figure 4.
Discussion
In this study, we describe two cases of TLE from a multimodal standpoint, with a view to determining the value of using multiple methods concomitantly. We first discuss the relationship between structural connectivity and lateralization of frontal activations that is revealed by picture naming. Then, we discuss possible links between functional/structural connectivity and cognitive performance.
As for Patients 1 and 2, about 30% of patients with focal and drug-resistant epilepsy exhibit atypical organization for language (e.g. partial or complete right hemispheric shift; Baciu and Perrone-Bertolotti [2015]) and verbal memory [Golby et al., 2002; Richardson et al., 2004]). The fact that this incidence is twice as much as observed in healthy subjects suggests that functional reorganization is induced by epilepsy (Baciu and Perrone-Bertolotti, 2015). In multiple studies, a correlation between functional reorganization and structural changes in TLE patients has been observed. For example, Labudda et al. (Labudda et al., 2012) showed that TLE patients with atypical language representation (right hemispheric dominance) exhibit an increased in grey-matter volume of the right temporo-lateral cortex, relative to TLE patients with typical left hemispheric dominance. Another study (concerning structural connectivity) found strong correlations between functional lateralization indexes obtained with fMRI for language on one hand, and asymmetry indices for structural connectivity on the other (James et al., 2015). Structural connectivity and brain function are closely related (Hervé et al., 2013).
In terms of structural connectivity in general and our study in particular, Patient 1 exhibited predominant left hemispheric impairment of multiple WM bundles (left Unc, ILF and Fox). This anatomical asymmetry, which is detrimental to the left hemispheric bundles, may be related to atypical representation (i.e. right hemisphere) of language, both probably accompanied by severe, chronic and (thus) untreatable epileptic seizures. However, other factors unrelated to epilepsy may explain the right hemispheric dominance of language in Patient 1, who was left-handed. In this regard, Mazoyer et al. (2014) found that 0.6% of the general population with right hemispheric dominance for language are left-handed. However, this category relates solely to strongly left-handed individuals, which Patient 1 was not (her Edinburgh quotient was -40%). In fact, more than 80% of left-handed individuals exhibit either left hemispheric lateralization (Szaflarski et al., 2012) or bilateral representation of language production.
Regarding Patient 2 (and unlike Patient 1), there was no significant anatomical asymmetry of WM bundles, given that impairment was observed in only one fascicle each in the left (Unc) and right (Arc) hemispheres. Interestingly, the body of corpus callosum (BCC) that links homologous pre-central areas was impaired in Patient 2. Given that the corpus callosum could play a more inhibiting role than a facilitator in the transfer of information between hemispheres (Levitan and Reggia, 2000; van der Knaap and van der Ham, 2011), its impairment may explain the bilateral frontal activation for language observed in this patient. This bilateral representation might be induced by reduced transcallosal inhibition between the frontal areas (Baciu and Perrone-Bertolotti, 2015). But here too, these explanatory hypotheses should be regarded as tentative only, given that this atypical pattern of language organization is not specific to epilepsy, but can also occur in healthy individuals (“ambilateral” or “symmetrical” pattern [Hervé et al., 2013; Mazoyer et al., 2014]). However, Patient 2 is strongly right-handed (Edinburgh quotient = + 90%) and the majority (95%; (Mazoyer et al., 2014) of right-handers exhibit typical left hemispheric lateralization for language. We therefore hypothesize that the bilateral functional representation in Patient 2 may be consecutive to epilepsy.
As for the correlation between anatomo-functional reorganization patterns and cognitive performance in TLE patients, it has been found that (1) diminished cognitive performance is ascribable to atypical functional organization of language (Bonelli et al., 2011); and (2) the integrity of subcortical microstructures correlates strongly with performance (Kucukboyaci et al., 2014). In addition, there is now a significant body of evidence indicating a strong correlation between subcortical microstructure and cognitive scores (e.g. as observed in normal aging [Madden et al., 2009], Parkinson's disease [Zheng et al., 2014], and bipolar disorders [Oertel-Knöchel et al., 2014]).
In our study, the changes in Patient 1's WM bundles mainly relate to memory function (bilateral Unc, left ILF and Fox). In TLE patients, the integrity of the left Unc usually correlates with verbal memory performance (Diehl et al., 2008), whereas left ILF and Fox correlate with immediate and delayed memory (Riley et al., 2010). However, even though Patient 1's memory scores are non-pathological, they are below average. We therefore hypothesize that in Patient 1, the degree of subcortical injury and the intensity of neuropsychological deficit may exist on a continuum and that this patient's WM bundle impairment may not have been severe enough to provoke a major cognitive disorder. Cognitive deficits in TLE patients are mild to moderate as a rule and are likely to result from a steady process of anatomical-functional reorganization extending over a number of years - a process that is in turn related to the chronicity of epilepsy (Keller et al., 2012). In Patient 1, this phenomenon may be attributable to the fact that she compensated for her cognitive deficit (mainly during verbal memory tasks) by recruiting right-hemisphere areas. In addition, Patient 1 had clearly pathological scores on the working memory test involving more robust executive components, since working memory entails mental manipulation of the material to be memorized (Baddeley, 2003). Moreover, the neuropsychological assessment of Patient 1 mainly revealed executive abnormalities (tendency to persevere, lack of mental flexibility). In that respect, Patient 1's pattern of cognitive performance could mainly be attributable to the impairment of bilateral frontal sub-cortical WM bundles.
The main deficit observed in Patient 2 is related to phonological and lexico-semantic processing. Such language impairments may be induced by a decline of executive functions, among other factors (Baciu et al., 2016). However, this type of decline is unlikely to be the cause of Patient 2's cognitive deficits, given that her executive performance was not pathological. Moreover, although phonological fluency engages executive functioning (Shao et al., 2014), naming mainly entails lexical access and lexico-semantic representations (Baciu et al., 2016). Patient 2's speech language assessment revealed lexical and phonological deficits (production without comprehension disorder [Tran, 2007]), mainly characterized by a lexical access deficit. This was a not insignificant impairment for Patient 2, given that she complained of its impact on her daily life (difficulty in finding words in conversations: the tip of the tongue phenomenon). Patient 2's pathologies may instead be attributable to the atrophy and blurring of the left temporopolar region that was revealed by MRI. This concept is supported by the fact that the temporal pole is regarded as a central hub of multiple networks involved in language (Mesulam, 2000), particularly when it comes to accessing verbal labels (Grabowski et al., 2001). Thus atrophy and blurring of the temporal pole can accompany lexical-access deficits (Olofsson et al., 2013). This observed cognitive pattern is also consistent with the structural connectivity findings for Patient 2, given that she exhibited isolated impairment of the left uncinate bundle, which links the orbitofrontal region to the temporal pole. The cognitive disorders observed in Patient 2 are probably attributable to the impairment of both cortical and subcortical structures. Patient 2 also exhibited bilateral activation of the regions involved in naming, in general and lexical access in particular. Thus, it appears that this type of bilateral reorganization is inefficient and does not help to maintain normal performance.
Thus, to sum up, the cognitive deficits observed in Patient 1 could be attributable to the impairment of intra-hemispheric WM bundles. The patient exhibited no language deficit but did show atypical functional (re)organization with right hemispheric dominance, which appeared to be cognitively efficient for language. As for Patient 2, the involvement of intra-hemispheric bundles and inter-hemispheric fibres via the corpus callosum may be the cause of her language deficits. In this patient, functional reorganization (bilateral representation) of activation appeared to be cognitively inefficient for language production.
Cases studies provide insight into the richness of neurocognitive functioning based on the kinds of patient particularities that tend to be obscured by results from group studies. However, these studies are in fact very often qualitative and purely descriptive, such as ours, and therefore only allow for the establishment of indirect links. While the different data can be consistent between them, it is important to keep in mind that there are no statistics to show correlations between datasets. In addition, only longitudinal studies may be able to prove that the anatomical-functional reorganization is attributable to epilepsy. The issue of whether an atypical organization is the consequence of epilepsy or whether an atypical organization might predate the onset of epilepsy (or even its cause) remains to be resolved.
It should also be noted that interpreting the results of studies involving patients is often problematic given that, in addition to the inter-individual variability observed in all individuals (such as age, manual laterality, etc.), other factors directly related to pathology (such as location of the EZ, duration of epilepsy, frequency of seizures, etc.) may also need to be factored into the equation. It is important to note that Patient 1 and 2 presented significant differences in terms of age and epilepsy duration (even if the onset of epilepsy was almost the same). These differences could partly explain the results we obtained. Normal aging can modify the structure of the WM and these changes are frequently associated with age-related cognitive changes such as executive functioning (Hirsiger et al., 2017). The age of Patient 1 (52 years) may partly explain the change in FA, especially for left and right Uncinate fascicles (frontal tracts) and a decrease in executive performance. But this factor does not seem necessary and sufficient to explain such a large decline. However, the duration of epilepsy, shorter for Patient 2 (12 years versus 37 years for Patient 1) could be the cause of the non-effective bilateral functional reorganization. Conversely, a longer duration of epilepsy, as for Patient 1, could lead to a more substantial change in anatomical connectivity as well as a switch in the right hemisphere (Powell et al., 2007).
Our study may also be prone to certain methodological biases such as the use of a relatively small normative sample size for MRI - which would need to be increased in order to verify the robustness of the results. In addition, the study was carried out using a naming task for the evaluation of functional activations related to language and memory. While this task has the advantage of being easy to perform in a clinical setting and brings into play both language and memory, it omits factors such as the syntactic features of language. Further studies on other types of tasks would be needed to assess the reliability of results obtained from fMRI, structural connectivity, and cognitive scores. It would also be useful to evaluate anatomo-functional reorganization at a more detailed level from an intra-hemispheric or regional standpoint and not only at the hemispheric level, although intra-hemispheric reorganizations are more difficult to identify (Baciu and Perrone-Bertolotti, 2015).
Conclusions
The inclusion of multimodal data in clinical studies is currently a major challenge. Since the various datasets obtained from MRI neuroimaging and cognitive scores are probably interrelated, it is important to go beyond the mono-modal approach and move towards greater integration of several multimodal data. In point of fact, multimodal integration of anatomical, functional and cognitive data facilitates the identification of comprehensive neurocognitive patterns in epilepsy patients, thus enabling clinicians to differentiate between reorganization profiles of greater and lesser robustness for this pathology. This, in turn, would lay the groundwork for major clinical advances such as the identification of functional areas, the prediction of post-surgical outcomes for curative neurosurgery, and projecting the scope of, or optimizing, any rehabilitation that may be necessary - all this with a view to providing more individualized care.
Supplementary data
Supplementary tables are available on the www.epilepticdisorders.com website.
Disclosures
None of the authors have any conflict of interest to declare.
Ethical statement
Participants (controls and two patients) provided written informed consent to participate in the study, which was approved by the local ethics committee (CPP: 09-CHUG-14/ANSM (ID RCB) 2009-A00632-55).