Epileptic Disorders
MENUCumulative effects of antiseizure medication on intelligence in children with focal epilepsy Volume 24, numéro 5, October 2022
Epilepsy is one of the most common brain disorders in children, with a prevalence of approximately 1% of all children [1]. A major issue in children with epilepsy is cognitive comorbidity. The underlying pathology [2, 3] and epilepsy-related factors [4, 5] may cause this comorbidity, but an additional reason for concern is the possible side effects of antiseizure medication (ASM).
Many studies have demonstrated that children who are using ASM experience cognitive side effects [6-10]. These studies, however, did not look at any potential long-term effects. The only knowledge we have on potentially harmful effects of ASM use on neural development comes from two fields of research: rodent studies [9] and studies on cognitive outcome after prenatal exposure of children to their mothers’ ASM. ASM may affect the unborn child’s brain development during pregnancy and increase the risk of intellectual disability, language impairment, psychomotor decline, and autism spectrum disorders [9, 11-14].
Neurodevelopment, however, does not stop after birth. Critical processes take place between the ages of one and six years, such as maturation of cortical structures and establishing their connectivity [15]. Furthermore, dendrite growth, synaptogenesis, and myelination, amongst others, continue until adulthood [16]. Only one study has shown potential longterm effects of ASM on eventual intelligence quotient (IQ), which was a trial using phenobarbital in children with febrile seizures [17, 18]. However, this is not informative for current practice, as this drug is now rarely prescribed beyond the neonatal age. Because the mechanism of action of ASM includes many pathways that are also important in the brain’s developmental processes, we hypothesized that the use of ASM early in life can affect brain development and eventual intellectual functioning.
We tested this hypothesis with an assessment of the long-term effect of exposure to ASM on IQ as a broad measure of neurodevelopment. The secondary aim was to investigate whether the relation between ASM and IQ was mediated by cortical thickness and brain volume measures.
Methods
Study population
In this retrospective cohort study, data were obtained from children with epilepsy [19], evaluated and followed at our outpatient child neurology clinic, our first seizure clinic, and from those who were evaluated for epilepsy surgery between 2005 and 2017. Children were included when they had focal epilepsy according to the International League Against Epilepsy classification [20]. Additional inclusion criteria were the availability of a neuropsychological assessment (NPA) during or after medication treatment and a magnetic resonance brain (MRI) scan for measuring cortical thickness and volumes (T1-weighed 3D MRI at 1.5 or 3.0 Tesla). Both NPA and MRI were required to be performed between the age of five and 12 years. Children with NPA or MRI below the age of five were excluded because of the difficulty of reliable automatic segmentation of MRI due to poor grey-white matter differentiation and shape differences relative to adult templates in immature brains [21]. The first treatment, however, could have been administered before the age of five years. A maximum age of 12 years was chosen to limit the age span and reduce study population heterogeneity. To study the effect of ASM on the cortex, only children with focal epilepsy were included, confined to a single hemisphere, as based on semiology, (inter)ictal EEG findings, and in case of structural aetiologies, the exclusion of contralateral MRI-lesions. Furthermore, patients were excluded when there was a risk of a diffusely affected brain, for instance, patients with a history of generalized epilepsy, tuberous sclerosis complex, mitochondrial or another metabolic disease, or children who had suffered from epileptic encephalopathies at some point during their disease (box 1). Similarly, patients who had epilepsy requiring hemispherectomy were excluded. Patients with a history of oral steroid treatment or thiopental coma were excluded from analysis as these interventions may influence total brain volume. Lastly, patients were excluded when they underwent epilepsy surgery before NPA and acquisition of MRI, as epilepsy surgery itself impacts cognition [5].
The Dutch Medical Research Involving Human Subjects Act did not apply, as confirmed by the Ethical Committee of the University Medical Center in Utrecht. Patients with active treatment at this centre were approached to obtain informed consent. Patients who previously objected to being part of scientific research were excluded.
Medication load calculations
Cumulative medication load was calculated by adding the years each ASM was taken until the NPA. Since the effect of long-term exposure to ASM was our primary interest, rescue medication for acute seizures was not considered in this calculation. Medication load is defined in units of medication-years, where one medication-year corresponds to one ASM being taken for one year. As an example, when a child used both valproic acid and levetiracetam for one year during the timespan preceding NPA, the medication load was two medication-years. Data were also collected regarding the number of ASMs taken at the time of NPA and MRI as potential confounders.
Brain structure measurements
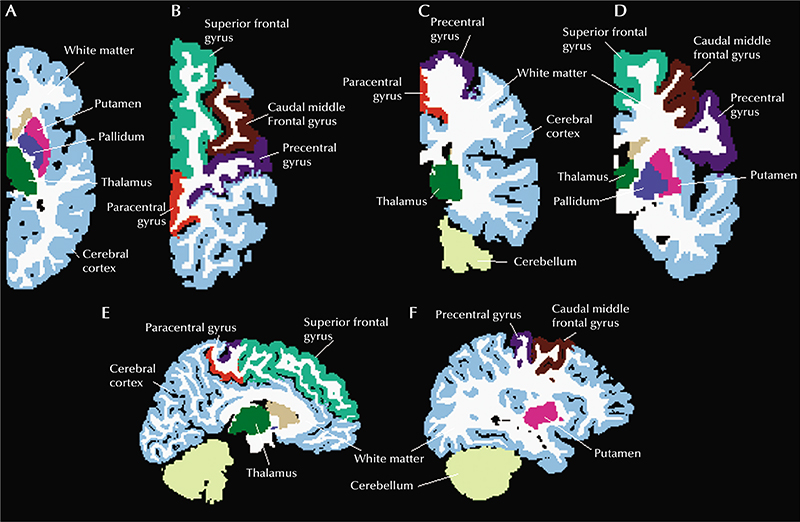
The MRI sequences were visually inspected for quality, and scans with excessive artifact precluding automated processing were excluded. Scans were segmented and parcellated using Freesurfer Version 5.3.0 [24]. Cortical thickness [25] was calculated at each surface vertex as the distance from the grey-white boundary to the cortical surface using the regions of the Desikan-Killiany atlas [26]. All segmentations and parcellations were manually checked for accuracy. We made manual adjustments to registration, parcellation and segmentation if necessary. Only measurements from the hemisphere from which seizures did not arise were considered. The mean cortical thickness of the entire healthy hemisphere, contralateral to the seizure focus, and several specified cortical regions were analysed. The precentral gyrus, caudal middle frontal gyrus, paracentral gyrus, and superior frontal gyrus were specifically investigated because cortical thickness in these regions has been reported to be most affected in people with epilepsy [27]. Cerebral volume changes were studied, investigating total hemisphere volume and the ratios between white matter volume / total hemisphere volume, and cerebellar volume/total hemisphere volume. Volumes of thalamus, pallidum and putamen were also analysed, following the findings described by Whelan and colleagues [27]. Regions analysed are displayed in figure 1.
Box 1 Definitions.
Aetiology of epilepsy: genetic, structural, metabolic, immune, infectious, unknown; according to International League Against Epilepsy classification [22].
Duration of active epilepsy: calculated as the difference between the date of first seizure and the date of last seizure expressed in years. Date of NPA or MRI was used when completed prior to the last seizure, or when the date of last seizure was unavailable.
Maximum seizure frequency: defined as the highest seizure frequency as noted in the patient’s history before NPA was performed, expressed in seizures per day.
History of secondary generalised seizures: scored positively if a secondary generalised seizure was reported.
History of status epilepticus: scored positively if any case of status epilepticus requiring hospitalisation was reported. Status epilepticus was classified according to the International League Against Epilepsy guidelines [23].
Endpoints of interest
The primary endpoint was IQ, which was assessed in children who underwent NPA for clinical reasons at the treating physician’s discretion. Whenever multiple NPAs were available, results from the latest assessment were included. A clinical child neuropsychologist carried out all NPAs. Each NPA resulted in standardized scores for total intelligence quotient (TIQ), verbal intelligence quotient (VIQ) and performance intelligence quotient (PIQ).
Data collection and analysis
Data were obtained from patient records and consisted of general patient characteristics, epilepsy characteristics, results of structural MRI as concluded by an epilepsy-dedicated neuroradiologist, detailed information on drug therapy, and results from NPA. Multiple factors influence cognitive epilepsy outcome [4, 5]: aetiology, maximum seizure frequency, duration of active epilepsy, age at onset of antiseizure treatment, history of secondary generalized seizures, history of status epilepticus requiring hospitalization and the number of ASMs used during NPA. Since these factors may also influence the medication load, they were predefined as potential confounders. Definitions of the confounders are listed in box 1.
We tested the relation between medication load and IQ univariably for the primary endpoint using linear regression to obtain a crude estimate of the association. Next, multivariable regression analysis was performed by adding a propensity score, computed using all possible confounders, to the analysis to obtain an adjusted regression coefficient. Children with IQ<70 are considered to have cognitive impairment; for sensitivity analysis, all patients with an IQ<70 were excluded and the analysis was repeated. Secondly, to assess the hypothesis that the effect of medication load on IQ was mediated by changes in cortical structures or brain volume, a mediation analysis [28] was performed in three steps as visualized in supplementary figure 1.
Statistical analysis was performed using SPSS Statistics (Version 25). For the primary analyses, p<0.05 was considered statistically significant. A Bonferroni correction for multiple testing was applied for the secondary analyses, resulting in an alpha level of 0.05/11 = 0.0045. Since sample size was limited, all multivariable regression analyses included propensity scores computed of all predefined possible confounders.
Results
We screened 1,658 children for eligibility, and finally included 59 patients (supplementary figure 2). Most frequent reasons for exclusion were no diagnosis of epilepsy (n=885), no MRI acquired in our centre (n=305), types of epilepsy other than focal (n=148), and no available MRI and NPA between the age of 5-12 years (n=51). Baseline characteristics of the study participants are presented in table 1. Median medication load (interquartile range [IQR]) was 5.3 medication-years (2.0-11.1) and the median duration of active epilepsy was 4.1 years (1.9-6.7). The timing of the MRI and NPA was closely related, with a median (IQR) duration of active epilepsy up to NPA of 4.1 years (1.7-6.7), and up to MRI of 4.1 years (1.9-6.7). The most commonly used drugs were valproic acid (57 patients), clobazam (38 patients) and carbamazepine (37 patients) (supplementary table 1). Mean (±SD) IQ scores were TIQ 77.4 (±18.9), VIQ 83.9 (±18.2), and PIQ 80.3 (±15.0).
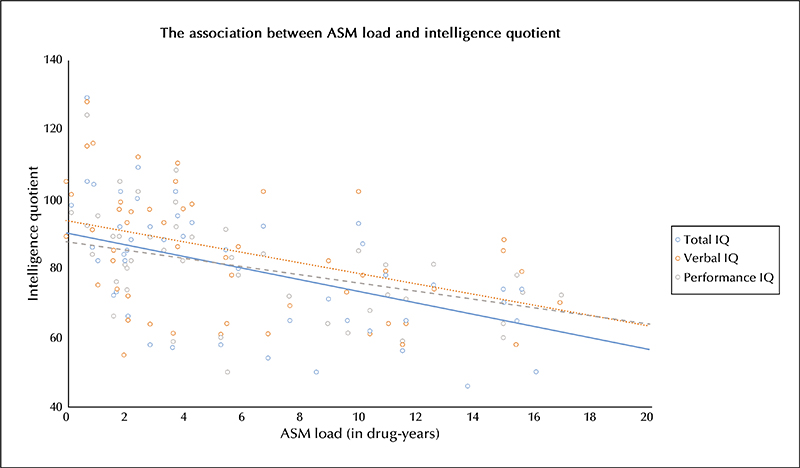
An increase in medication load was associated with a decrease in TIQ. This association was significant in both the crude linear regression (regression coefficient [RC]: -1.7 per medication-year;95% confidence interval [CI]: -2.2 to -1.1) and the adjusted model (RC: -1.2;CI: -2.0 to -0.3) (table 2). Crude analyses for both VIQ and PIQ showed that an increase in medication load was significantly associated with a decrease in VIQ or PIQ (figure 2) (VIQ: RC -1.5, CI -2.2 to -0.8;PIQ: RC -1.2, CI -1.9 to -0.5). This association remained marginally significant for only PIQ after adjustment for potential epilepsy-related confounders (table 2). Sensitivity analyses for patients with TIQ≥70 revealed a significant association for TIQ and PIQ in multivariable regression analyses (TIQ: RC -1.6, CI -2.9 to -0.3; PIQ: RC -2.2, CI -3.5 to -0.9), although not for VIQ (RC -1.0, CI -2.6 to 0.7) (supplementary table 2). In supplementary table 3, the effect of ASM load is broken down into medication load during monotherapy and medication load during polytherapy. The adjusted effects are similar to the main effects described in table 2, with a significant correlation between drug load and TIQ, VIQ and PIQ in children with monotherapy, and a significant correlation between drug load and TIQ in cases with polytherapy. Linear regression analyses between medication load and brain thickness and volume measures did not reveal a significant association in crude and adjusted models with the Bonferroni corrected alpha level of 0.0045 (table 3). Additionally, no significant association was found between cortical thickness and volumes of individual brain structures and TIQ, VIQ and PIQ scores (table 4). The third step of the mediation analysis (supplementary figure 1) was not executed because the first two steps did not show any significant association between medication load and brain structure nor between brain structure and IQ. Interestingly, in the adjusted models, thalamus volume tended to correlate with IQ, only becoming significant for PIQ, after adjustment for other factors; RC 7.6 IQ points per mm3 volume increase (CI: 1.4 to 13.7; p = 0.02) (table 4). However, this variable did not cross the Bonferroni-corrected alpha level of 0.0045 and should therefore be considered non-significant.
Discussion
The main finding of this study is that higher cumulative ASM load is associated with lower eventual TIQ in children. This result remained significant after adjustment for epilepsy-related confounders, including the use of ASM at the time of NPA, suggesting that the observed association is explained by the cumulative effect of previously used medication itself. A similar, only marginally significant association was observed for PIQ. No significant association was found between medication load and cortical thickness or brain volume of the unaffected hemisphere, nor between cortical thickness or brain volume of the unaffected hemisphere and IQ. To illustrate the clinical implication of these results, the median medication load in the study population was 5.3 medication-years, which would translate to an average TIQ reduction of 6.1 points (CI: -10.7 to -1.6), which is not negligible in children. Future studies investigating whether there are any associations amongst ASM, IQ and subcortical volumes [29] or brain networks [30] in children with focal epilepsy would be of interest.
Whereas most studies have focused on direct cognitive side effects of ASM during use [6-10], the longterm effects of ASM exposure on cognitive function remain largely unknown. Farwell and colleagues studied the long-term effects of ASM on IQ in children in a relatively healthy cohort of children with febrile seizures [17]. In this trial, 217 patients with febrile seizures, aged 8 to 36 months, were randomized to a phenobarbital or placebo treatment arm and followed for two years. At the end of the trial, the average IQ score in the phenobarbital group was 8.4 points lower than in the placebo group (CI: -13.3 to -3.5). After discontinuation of the trial medication, the difference was still 5.2 IQ points (CI: -10.5 to 0.04), and 3-5 years after the trial, the children from the phenobarbital group scored lower on a Wide Range Achievement Test [18]. However, there was no longer any significant difference in average group IQ. Both the Farwell trial and our study suggest long-term effects of ASM on cognition, although both have limitations. In the case of the Farwell trial, results are based on intention-to-treat analyses, and many participants stopped trial medication or were lost to follow-up. Also, currently phenobarbital is not prescribed as a standard treatment against epilepsy beyond the neonatal period.
Recently, the ENIGMA-epilepsy consortium investigated structural brain abnormalities in epilepsy patients and healthy controls [27]. Compared to healthy controls, significantly smaller volumes of the thalamus, hippocampus, and pallidum of people with epilepsy were shown, and reduced cortical thickness was found across seven regions bilaterally. In our study, which did not include control subjects, we were unable to establish an association between structural changes in these regions and medication load or IQ. Because our study did not reveal an association between medication load and IQ based on changes in brain volume or cortical thickness, the pathophysiological mechanism behind the influence of ASM on IQ remains unclear. However, the absence of gross volumetric MRI changes certainly does not exclude subtle changes in cortical microstructure and connectivity, nor subcortical changes. Neurogenesis, apoptosis, synaptogenesis and pruning are all relevant processes in neurodevelopment [16, 31]. Disturbing these developmental processes might account for cognitive deficits in humans pre-or postnatally exposed to ASM [31]. The hypothesis is that these disturbances are related to decreased neuronal activity during development, since all ASMs share this effect [32].
Animal studies have given some insight into the possible neurotoxic effect of ASM. In rodent studies, it has been shown that some ASM trigger widespread apoptotic neurodegeneration throughout the developing brain when administered during the period of rapid brain growth [32]. Furthermore, it was suggested that neurotransmitters could modulate the proliferation of neural stem cells, neuroblasts and glioblasts, regulate migration and induce differentiation [33-36]. In this way, pharmacological interference of neurotransmission may influence brain development. Similarly, ASM may cause permanent defects in the central nervous system. These findings in animal studies suggest a potential influence of ASM on neurodevelopment in humans. The results of the current study seem to support the theories above.
A strength of this study is that it firstly provides new insight into long-term cumulative effects of ASM on intelligence, as previous research mostly focused on short-term adverse cognitive effects due to ASMs. Secondly, the effect was studied while adjusting for known confounders, including the number of ASMs used at the time of NPA to exclude the acute side effects of ASMs. Finally, the amount of missing data was limited, despite the retrospective study design.
A conclusion regarding a causal relationship between medication load and IQ can, however, not be drawn, as this study has several limitations. The study population consisted of a selected group of children, since a patient was only included when both NPA results and 3D T1 MRI were available. However, NPA will usually be performed in children with a suspicion of cognitive deficits (following Dutch Guidelines [37]) or with severe epilepsy, possibly excluding less affected children from the study, leading to potential selection bias. The finding that general IQ scores were remarkably low in our cohort supports this theory. The lower IQ scores were not explained by the aetiology of epilepsy, which was most frequently structural, but it is a potential indication of a selected cohort. This could have resulted in an overestimation of the relationship between medication load and IQ. However, subgroup analysis of patients with TIQ≥70 still revealed a significant, negative association for medication load and TIQ, supporting the claims of the main analysis.
As NPAs before start of drug treatment were unavailable, no longitudinal comparison at the individual level was possible. Also, results from a control group of epilepsy patients without antiseizure medication were unavailable, complicating correction for unknown confounders. Additionally, no distinction was made for different ASMs or dosage, as the objective of the current study was to explore the relationship between overall ASM load and IQ and the sample size was too small to allow for analyses of ASM subgroups. Because the mechanism of action of each antiseizure medication is different, the effects of each drug on neurodevelopment might be very different. They should ideally be studied separately or in consort with drugs using the same mechanism.
Furthermore, a methodological limitation is the relatively small overall sample size, with unequal sizes of aetiology subgroups. Finally, studies investigating the influence of epileptic seizures on IQ, while correcting for ASM use are scarce. Therefore, separating the effects of long-term medication use from those of ongoing seizures remains difficult.
Altogether, the observed relation must be confirmed in a prospective study with a representative sample from the whole epilepsy population while assessing the effect of ASM in different stages of neurodevelopment and exploring these effects for different subgroups of medications.
Conclusion
This study revealed a significant negative relation between ASM load and TIQ after adjustment for epilepsy-related confounders and the number of ASMs taken at the time of NPA. Changes in MRI volumetry and cortical thickness measures did not mediate this relation. Since the implications of the current study are considerable, the results have to be interpreted with caution and confirming a possible causal relation between medication load and IQ in a prospective, longitudinal study design is desirable. As a concluding remark, it must be mentioned that a potential effect of ASM may very well be justified compared to the detrimental effects of ongoing seizures.
Key points
- This study provides new insight into long-term cumulative effects of antiseizure medication on intelligence in children with epilepsy while correcting for the effects of epilepsy itself.
- Higher antiseizure medication load is associated with lower total IQ.
- This was not associated with underlying changes in brain thickness and volume.
Test yourself
- How was cumulative antiseizure medication load calculated in this study?
- Cumulative medication load was calculated by adding the number of ASMs that were taken during the follow-up period.
- Cumulative medication load was calculated by adding the years each ASM was taken, corrected for dosage, until the NPA.
- Cumulative medication load was calculated by adding the years each ASM was taken until the NPA.
- This study evaluated whether the relation between ASM and IQ was mediated by changes in cortical thickness and brain volume. What were the specific brain areas studied?
- Cortical thickness was evaluated in the superior frontal gyrus, precentral gyrus, caudal middle frontal gyrus and paracentral gyrus.
- Cortical thickness was evaluated in the thalamus, pallidum, putamen and superior frontal gyrus.
- Cerebral volume was evaluated for total brain volume, white matter volume, cerebellar volume, thalamus, putamen, pallidum and hippocampus.
- How should the relation between ASM load and total IQ be interpreted?
- An increase of ASM load by one medication-year resulted in a decrease in total IQ of 1.2 points.
- When the number of ASMs increased by one, total IQ decreased by 1.2 points.
- When a child was on two ASMs during a period of one year, our study results revealed a decrease in total IQ of 1.2 points.
Note: Reading the manuscript provides an answer to all questions. Correct answers may be accessed on the website, www.epi/epticdisorders.com.
Supplementary material
Supplementary data and summary slides accompanying the manuscript are available at www.epilepticdisorders.com.
Acknowledgements and disclosures
We would like to acknowledge the Epilepsiefonds for their financial support under grant number WAR 08-10. Julie Woodfield held a Wellcome Trust Fellowship through the Edinburgh Clinical Academic Track (ECAT) scheme (106364/Z/14/Z). For the purpose of open access, we will apply a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
The authors have no financial relationships or conflicts of interest relevant to this article to disclose.
Availability of data and material
Anonymized data generated and analysed during the current study are available from the corresponding author on reasonable request.
Code availability
A statistical code will be shared with any qualified investigator by emailing the corresponding author.
Consent to publication
Patients with active treatment at the University Medical Center in Utrecht, the Netherlands, were approached to obtain informed consent. Patients who previously objected to data publication were excluded.