European Journal of Dermatology
MENUOral intake of a new full-spectrum hyaluronan improves skin profilometry and ageing: a randomized, double-blind, placebo-controlled clinical trial Volume 31, numéro 6, November-December 2021
Skin is the largest organ of the human body and represents the main barrier to the external environment [1]. It is exposed daily to several intrinsic and extrinsic factors which affect its ageing process [2]. Intrinsic factors include, above all, genetic susceptibility, skin pigmentation (protective against photo-ageing) and lifetime hormonal variations (especially in terms of oestrogen and testosterone). Among extrinsic factors, UV light exposure is the most affective ageing factor, accounting for more than 90% of visible skin photo-ageing due to collagen and elastin deterioration in the dermal extra-cellular matrix (ECM) [3]. Skin ageing is further influenced by concomitant lifestyle-related factors, such as ambient temperature, correlating with skin water loss and pollution. Diet and physical activity even contribute to ageing [4], along with chronic stress and glucocorticoid release [1] and smoking, which decreases capillary blood flow to the skin and increase free radicals and protein denaturation. Acting simultaneously, all these factors lead to structural and functional alterations of the skin [2], resulting in histological changes reflected by skin ageing signs, such as dryness and roughness, the appearance of wrinkles and pigmented spots, and decreased barrier function [3, 5].
The main skin components are collagen, elastin fibres and hyaluronan (HA); the former two account for roughly 97.5% and 2.5% of the components in the dermis, respectively. Collagen maintains the 3D structure of the skin, whereas elastin fibres form a 3D network between collagen [6]. With a capacity to bind up to 6000 times its volume in water, HA provides structure to the ECM, maintaining moisture and firmness [7]. In senile skin, HA is still present in the dermis, while in the epidermis it entirely disappears [7]. Even collagen and elastin content decrease through ageing, and skin gradually loses its mechanical tension [2].
Over the years, scientific research on skin has evidenced a set of molecules that are effective in preventing and treating ageing signs and wrinkles. HA is one of the most studied, due to its wide range of positive effects. HA is a linear, flexible, high-molecular-mass natural polysaccharide, belonging to glycosaminoglycans (GAG), consisting of alternating disaccharide monomers of N-acetyl-D-glucosamine and D-glucuronic acid, joined by β-1,4 and β-1,3 glycosidic bonds. Generally called hyaluronan, in humans, HA is present as hyaluronates, which are sodium, calcium, magnesium or potassium salts with polar groups that provide the macromolecule with its characteristic high viscoelasticity, biocompatibility, hygroscopicity and water retention capacity. Initially obtained from animal sources (e.g. rooster comb), HA is nowadays produced by bacterial fermentation of Streptococcus zooepidemicus, leading to the purest grades [8].
Considering that HA content in the skin of a 75-year-old person is less than a quarter of that of a 19-year-old subject, the need for HA supplementation is evident in order to compensate for HA deficiency [9]. Regarding its ability to replenish moisture in the skin, and considering its positive effect on endogenous collagen synthesis through dermal fibroblast stimulation and antioxidant effects, HA is of significant potential in nutricosmetics. Indeed, it is reported to improve skin hydration, smoothness and elasticity, as well as slow down wrinkles and deep line formation when topically used [10], however, there is a lack of bibliographic data on bioavailability and consistent efficacy based on oral intake.
For these reasons, the present study aimed to evaluate skin anti-ageing effects of an innovative hyaluronan food supplement, based on full-spectrum hyaluronan (FS-HA), produced through innovative technologies. Featuring a large spectrum of HA molecular weights, the active nutraceutical was administered to adult female Caucasian healthy subjects at a daily dosage of 200 mg in a double-blind, randomized, placebo-controlled clinical study for a period of four weeks.
Materials and methods
Human volunteers and study design
The single-centre, randomized, double-blind, placebo-controlled, parallel group study was carried out at Complife Italia Srl facilities in compliance with the Helsinki Declaration (1964) and its amendment. Study protocol and informed consent form were approved by the Independent Ethical Committee for Non-Pharmacological Clinical studies (Genova, Italy).
Sixty Caucasian healthy female subjects, aged between 35 and 70 years, showing mild-to-moderate signs of skin ageing, were enrolled according to inclusion and non-inclusion criteria (table 1), verified by a dermatologist. Based on an appropriate statistic algorithm (Wey's urn), subjects were randomly and equally assigned to active and placebo groups, and received a 200-mg daily dose of FS-HA (ExceptionHYAL® Star from Roelmi HPC - a novel FS-HA, produced through advanced technologies and characterized by a large spectrum of molecular weights, as well as maltodextrin) or the placebo (maltodextrin) for 28 days, in the morning with a glass of water, not around meal-time.
Before starting the study, eligible participants signed an informed consent reporting detailed information about the study. Enrolled subjects received the assigned product, together with a base cream to use on the face (as a substitute for normal routine beauty products, to be used twice a day - morning and evening), a self-assessment questionnaire and a planning of visits at the medical centre for parameter measurements: at baseline (T0d) and after 28 days of treatment (T28d).
Furthermore, an explorative study on a restricted number of subjects was carried out in order to evaluate the serum level of hyaluronic acid (HA): 10 subjects from each arm were randomly selected for venous sampling, to be performed before the first intake of product (T0d) and at 7 (T7d), 14 (T14d), 21 (T21d) and 28 (T28d) days of treatment.
Assessment of clinical effects
All clinical parameters were assessed by a dermatologist under controlled room conditions (T = 22 ± 2 °C and RH = 40-60%) after a 15-20-minute period of acclimatization.
Skin profilometry
Skin profilometry was assessed using Primos 3D (GFMesstechnik GmbH), a non-contact in vivo skin measurement device based on structured light projections that is capable of quantitatively evaluating skin surface properties and measuring wrinkle depth and wrinkle volume.
Skin hydration
Skin moisturizing evaluation was performed by the internationally recognized Corneometer® CM 825 (Courage + Khazaka Electronic, Köln, Germany) method, in which an instrumental probe shows changes in capacitance according to moisture content of the skin, on the basis of the different dielectric constant of water and other substances.
Transepidermal water loss (TEWL)
Transepidermal water loss (TEWL) was measured using TEWAMETER 300® (Courage + Khazaka Electronic, Köln, Germany) according to the internationally recognized TEWAMETER® method.
Skin elasticity and skin firmness
Skin elasticity and skin firmness were assessed on the face (cheek) with the Cutometer® MPA 580 (Courage + Khazaka Electronic, Köln, Germany). Skin elasticity was calculated as the ratio between residual deformation (Uf) and maximum elongation of the skin (Ua); such a R2 ratio (Uf/Ua) indicates the ability of the skin to return to its original state of recovery after a stressful event: the closer the value is to 1, the more elastic is the skin. Parameter R0 = Uf, which may imply skin firmness, was also evaluated. A reduction in R0 parameter indicates an improvement in the ability of the skin to protect against deformation imposed by the probe during the suction phase.
Blood concentration of HA
HA serum levels were determined for the selected 20 subjects using a CLIA method (MAGLUMI HA from Shenzen New Industries Biomedical Engineering Co Ltd–CHINA) on blood samples collected before the first intake of the food supplement, and then with a weekly frequency up to the end of the study.
Self-assessment questionnaire
Subjects were asked to complete a questionnaire to express their personal opinion about the study product.
To evaluate treatment efficacy and tolerance, 10 questions were asked which referred to changes in skin properties and general aspects, appearance of wrinkles, product compliance and interest in taking the product further and pursuing the treatment. Subjects could answer “Agree/Disagree” to each question, thus agreement indicated a positive response to the above-mentioned variables.
Statistical analysis
Sample size was determined by assuming a mean reduction of wrinkle depth of 15 μm in the active arm and 7 μm in the placebo arm (with standard deviation of 7, effect size = 0.71), with α level of significance of 0.05 and a statistical power of 0.80. To account for a potential 10% drop-out rate, a total of 60 patients (allocation ratio = 1) were enrolled in the study.
Data were analyzed and interpreted according to both descriptive and inferential statistical analytic procedures. Instrumental data were analysed using the Student t-test for paired data (intra-group analysis) and unpaired data (inter-group analysis–T0 active group vs. T0 placebo group and % variation active group vs. % variation placebo group). Variations were statistically significant when p < 0.05. Serum HA levels were analysed using RM-ANOVA, followed by the Tukey-Kramer test. NCSS 10–PROFESSIONAL (vers. 10.0.7) was used as statistical software to elaborate the resulting data.
Regarding the self-assessment questionnaire, the percentages of subjects who attributed a given score to a given question were calculated. For each question, the number of subjects related to each response was counted and this number was then divided by the total number of subjects. The response was validated if at least 60% of subjects gave positive answers.
Results
The clinical study was carried out from September to November 2020. FS-HA and placebo treatments showed good compliance and were well tolerated by all subjects. In addition, according to the clinical assessments carried out by the dermatologist, no side effects or adverse events were reported throughout the study, and no drop-outs.
Features of subjects from the active and placebo groups related to age and skin phototype, type and sensitivity are presented in table 2, confirming the unbiased randomization.
Skin profilometry
Skin surface pattern was quantitatively analysed to assess wrinkle volume and wrinkle depth in order to evaluate skin profilometry evolution over the active treatment period. Results are shown in figure 1.
FS-HA treatment resulted in a significant decrease in wrinkle depth and wrinkle volume, both compared to baseline and the placebo-treated group. A -18.8% mean reduction in wrinkle depth was appreciated after 28 days of FS-HA intake; from a baseline value of 293.41 ± 21.27 μm to a T28d value of 232.19 ± 15.96 μm (p < 0.001 vs. baseline and placebo), with a maximum registered reduction value of -44.6% (from 566.30 μm to 313.90 μm). A smaller and non-significant reduction, -2.6%, was measured in the placebo group; from a T0d value of 230.50 ± 19.42 μm to a T28d value of 225.78 ± 20.87 μm.
A similar effect was achieved based on evaluation of wrinkle volume evolution; a -17.6% reduction in wrinkle volume was measured after 28 days of FS-HA intake, from a baseline value of 2.82 ± 0.37 mm3 to a T28d value of 2.31 ± 0.30 mm3 (p < 0.001 vs. baseline and placebo), with a maximum registered reduction value of 58.0% (from 0.98 mm3 to 0.41 mm3).
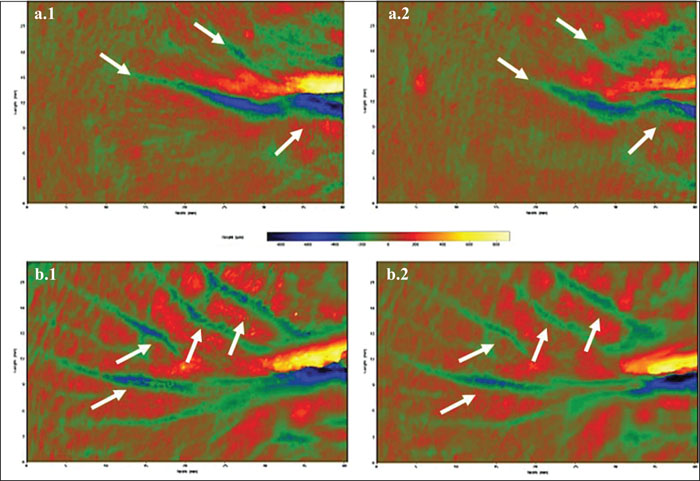
No statistically significant variations in wrinkle volume were appreciated in placebo-treated subjects.Figure 2 shows examples of Primos 3D images of two subjects treated with FS-HA, taken at baseline and after 28 days.
Skin hydration
Skin hydration values recorded at baseline for the FS-HS group showed a statistically significant increase by +10.6% (p < 0.001 vs. baseline, p < 0.01 vs. placebo), from 39.73 ± 1.61 c.u. (corneometric units) at T0d to 43.95 ± 1.85 c.u. at T28d, with a maximum registered value of +20.5% (from 40.0 c.u. to 48.2 c.u.). As shown in figure 3, a smaller but non-significant improvement (+1.4%) of skin hydration values was appreciated in the placebo group; from 44.41 ± 1.26 c.u. at T0d to 44.89 ± 1.74 c.u. at T28d, with a maximum registered value of +35.1%. However, the FS-HA group showed no negative differences (minimum: 0.2%) indicating a general improvement in the parameter, whereas reduction of skin hydration values was recorded in the placebo group with a minimum recorded variation of -28.0%.
Transepidermal water loss (TEWL)
FS-HA intake resulted in a significant (p < 0.001 vs. baseline) decrease in TEWL, from 8.87 ± 0.25 g/h/m2 at T0d to 8.52 ± 0.24 g/h/m2 at T28d, with a -3.9% reduction and a maximum recorded TEWL decrease of -14.3%.
As shown in figure 3, no significant variations were detected in the placebo-treated group, which showed instead increased TEWL by 1.6% (from 8.35 ± 0.43 g/h/m2 at T0d to 8.46 ± 0.45 g/h/m2 at T28d). TEWL reduction in the FS-HA group was statistically significant compared to the placebo-treated group (p < 0.01).
Skin elasticity–R2 parameter
Overall elasticity values (R2) increased by +3.8% in the FS-HA group, from a mean value of 0.679 ± 0.008 at T0d to 0.704 ± 0.007 after 28 days of treatment, as shown in figure 4. This increment was statistically significant compared to both baseline (p < 0.001) and the placebo-treated group (p < 0.01). In fact, the placebo group showed a decrease in skin elasticity by -2.7% (from 0.604 ± 0.015 to 0.586 ± 0.017), although this was not significant, with a minimum recorded value of -28.9% and a maximum recorded value of 25.4%. No negative differences (minimum: +1%) were recorded for the food supplement group.
Skin firmness–R0 parameter
FS-HA treatment resulted in a significant decrease in the R0 parameter by -5.1% (p < 0.001 vs T0), from a baseline mean value of 0.378 ± 0.006 Uf to 0.359 ± 0.006 Uf after 28 days, with a maximum value recorded of -11.7%. As per figure 4, no significant variations were found in the placebo-treated group, for which R0 was approximately the same; from 0.371 ± 0.011 Uf at T0d to 0.366 ± 0.011 Uf at T28d (-0.4% variation). Furthermore, the FS-HA group showed no reduction in skin firmness in all subjects, whereas this parameter showed both positive and negative variation in the placebo group, with greatest variation of +36.7%.
Blood concentration of hyaluronic acid
In vitro tests performed in order to evaluate HA bioavailability through reconstructed intestinal epithelium showed a 93.6% absorption rate after 24 hours of application of FS-HA (unpublished data).
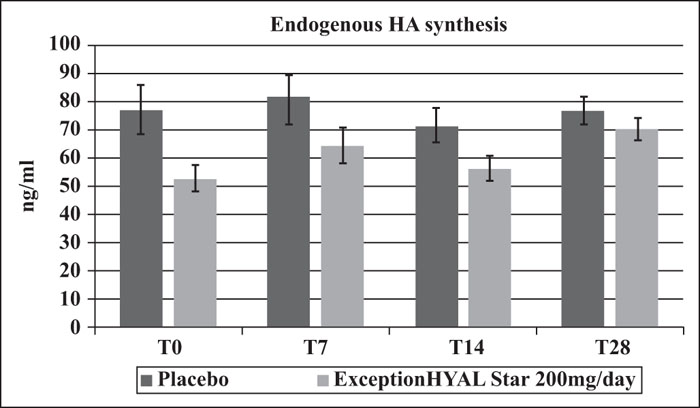
Serum levels of HA showed a wide distribution throughout the study, either between volunteers or within the same volunteers. However, a slight but progressive increment of mean HA was appreciated in the FS-HA group, though not significant, showing a 40.3% increment after 28 days of treatment (from 53.02 ± 4.52 ng/mL at T0d to 70.34 ± 3.78 ng/mL at T28d); mean serum levels of HA remained almost the same throughout the study in the placebo group (figure 5).
Self-assessment
After 28 days of treatment, subjects were asked to express their opinions on treatment efficacy and tolerance by answering “agree/disagree” to a questionnaire including 10 different questions, aimed at assessing subjects’ perception related to skin changes due to the treatment. The tested product was perceived to be effective and pleasant to use by at least 60% of the subjects. The FS-HA group declared that the administered product helped to make the skin more hydrated (93%), softer (90%) and smoother (87%); skin tone and elasticity were reported to be improved by 83% and 90% of subjects, respectively. Face wrinkles became less visible for 63% of FS-HA-administered subjects and 90% of participants stated that the product helped to improve general aspects of facial skin. Whereas less than 50% of participants in the placebo group positively judged product performance, overall, 93% of FS-HA-treated subjects expressed willingness to buy the product and pursue the treatment.
Perception of treatment efficacy was clearly better in subjects who took the product compared to those treated with the placebo. The product was well tolerated by individuals involved in the trial during the administration period.
Considering questions related to perception of product efficacy and responders as those who responded positively to five of the total seven questions, the FS-HA treated group showed 26 responders, whilst only 10 were documented in the placebo group.
Discussion
Skin health has been one of the most significant nutricosmetic focuses of recent years. The main components of skin are collagen, elastin fibres and HA that synergistically support its structure and allow it to appear smooth, moisturized, firm and shiny, with an appropriate defence system and ability to self-repair [1]. Through ageing, the level of these structural components is physiologically reduced and the epidermal HA content decreases from 0.03% in women aged 19 to 47 years down to 0.015% in women aged 60 years, and is halved to 0.007% in 70-year-old women [7].
HA is a GAG present in the skin and is responsible for moisturizing it, due to its water retention capacity. Considering its antioxidant effects and ability to stimulate endogenous collagen synthesis [10], HA is a key molecule in skin ageing treatment. Widely spread within different human tissues, it is present with different molecular weights, each with a specific role in biological processes [11].
The efficacy of a combination of a wide range of HAs of different molecular weight is greater when compared to a single specific HA [12]. In this context, the use of a new hyaluronan, FS-HA, characterized by a full spectrum of molecular weights (obtained through precise modulation of time, temperature and pressure during the bio-fermentation process), could mimic what physiologically occurs inside the body in terms of cell activation and response, soft tissue composition and allowing cells to react in a more sensitive way, together with endogenous HA-induced self-regeneration [13].
Oral treatments to slow down and reverse ageing processes fall into the nutricosmetic category and account for active products such as HA, hydrolysed collagen peptides, elastin and other substances that are effective against both free radical species and ECM deconstruction [2, 4]. Ingested HA is reported in the literature to positively affect skin health, and nutricosmetics may be active over a short time frame when used as an alternative, or in addition, to cosmetic treatments. However, reports in the literature are so far only based on clinical trials with long administration periods and high dosages, providing weak data [14-16]. In addition, reported studies often present a small sample size, lack a control group, and are open-label, resulting in less robust data due to inconsistent study design [7, 17].
Considering the scarce evidence from previous studies on the effects of traditional HA of animal or bacterial origin on skin ageing when taken orally, this study has been designed to clinically assess the improved effect of the oral supplementation of FS-HA on skin health during a short period (28 days) relative to a placebo group.
Regarding the assessment of hyaluronan serum levels after oral intake, the blood concentration showed an increase of 40.3% in the active group when compared to basal values, which was higher than that of the placebo group. Despite the fact that the results were not statistically significant over any of the experimental time frames compared to either placebo or baseline, due to preliminary evaluation based on a small sample size (n = 20), these results are promising. Indeed, HA bioavailability via oral intake has been shown to be very low; the level of absorption appears to be low with lower-molecular-weight (50-200 kDa) HA, while higher-molecular-weight HA shows an upward trend in blood concentration after 10 hours from administration [13]. Molecular weight is reported to be a key factor regarding the extent of HA absorption, which is dose-dependent and inversely proportional to molecular weight [18]. On the other hand, the increase in HA blood concentration in the FS-HA group appeared more likely to be independent of absorption rate and molecular weight distribution, and rather due to an enhancement of its physiological endogenous synthesis.
The outcomes of skin profilometry confirmed the effectiveness of FS-HA administration in slowing down and reversing skin ageing processes. Both wrinkle depth and volume showed a statistically significant reduction by -18.8% and -17.6%, respectively (vs. baseline and placebo; p < 0.001). Results after only four weeks with FS-HA consistently improved when compared to data collected for topical and oral administration of standard HA in terms of timing and effect.
The decrease in wrinkle depth and volume reflects a renewed structure of the skin, affected by a lack of collagen and elastin which causes skin thinning and an appearance of roughness. The greater elasticity suggests a reconstituted ECM due to new endogenous molecular synthesis and HA antioxidant activity, as reported in the literature [10]. The absorption rate of FS-HA through the intestinal epithelium was increased to over 93%, which appears to further highlight the biological affinity between FS-HA and human tissues. FS-HA confers enhanced cellular sensitivity, and it is the spectrum of molecules with different molecular weight that allows nutricosmetic targets to be achieved with maximum efficacy profiles [12]. FS-HA treatment also increased the skin's ability to retain its shape and contours on the face, appearing firmer and more distended. Lines and furrows typically emerging through the ageing process [1] were visibly reduced.
Test results on skin hydration revealed a significant improvement (+10.6% vs. baseline, p < 0.001; vs. placebo, p < 0.01) when compared to placebo (+1.4%) and reflected the efficacy of FS-HA in promoting skin hydration within only four weeks. As reported in the literature, HA is effective because of its water retention capacity and the stimulation of synthesis of endogenous structural molecules [2, 10, 19]. The improvement with FS-HA was probably due to its wider spectrum of molecules of different molecular weight, with an activity that mimics that of connective tissue. The daily administered dose in this study (200 mg) is in line with the literature, and the number of involved subjects in the present study is higher compared to the average number of enrolled patients in other trials [19]. Most of the studies in the literature were performed on Asian people, especially Japanese. Considering the importance of ethnicity on intrinsic factors of skin ageing, another strength of this study is the Caucasian origin of subjects.
Recovery of skin elasticity was measured by Cutometer®, resulting in statistically significant improvement of R2 values with respect to both baseline (p < 0.001) and placebo (p < 0.01). Furthermore, evaluation of skin firmness, related to R0 (Uf, corresponding to the maximum elongation of skin), reflected a statistically significant positive variation when compared to baseline (p < 0.001). This is another reference value in skin health assessment, which is inversely proportional to the degree of skin compactness; indeed, the lower the R0, the greater the skin's ability to protect against deformation imposed during the suction phase. Oral consumption of FS-HA significantly improved viscoelastic properties of the skin, confirming its ability to restore ECM through stimulation of endogenous HA synthesis.
Considering the recorded variation in skin moisture, elasticity and firmness, and comparing the maximum and minimum data points at both baseline and T28d for each group, the data for FS-HA-treated subjects was consistently more favourable compared to that of placebo-treated subjects. This suggests that the FS-HA-based supplement, as well as being an effective anti-ageing treatment, also supports skin health and preserves the skin.
The measure of TEWL is used to assess skin barrier function and its capacity not to dehydrate, especially when exposed to dry and cold environments. In FS-HA-treated subjects, TEWL significantly decreased when compared to both baseline (p < 0.001) and placebo (p < 0.01), however, TEWL instead increased by +1.6% in the placebo group. These changes reflect the efficacy of nutraceutical ingredients in supporting skin barrier functions, which are further compromised throughout ageing by physiological changes in lipid, salt and amino acid profiles in the ECM [4, 19]. The decrease in TEWL reflects the potential effect of FS-HA in supporting the reconstitution of aged skin, by promoting cell proliferation to fill the gaps of skin cells and increasing the amount of HA synthesis in the skin, as observed by Kawada et al.
Finally, self-assessment questionnaire outcomes confirmed the positive analytical results. The subjective evaluation of product performance and whether it was pleasant to use reflected the perception of improvement of healthy skin indicators in the active group, with respect to the placebo group. The overall improvement in skin parameters was reported by more than 90% of subjects and the reported product tolerance and compliance confirmed the safety and non-sensitizing and non-allergenic properties of FS-HA used as a nutraceutical.
In conclusion, hyaluronan is ubiquitous in the body, and is present in the form of different molecular weights which activate very different biochemical pathways. In vitro and ex vivo studies have proven that HA polymers show different effects according to their molecular weight. While several studies report HA anti-ageing effects with topical applications, few data are available on oral intake. To address this, this study reported clinical results in terms of absorption and efficacy of FS-HA, an innovative hyaluronan for oral use, characterized by a large spectrum of molecular weights that match different biological needs. Oral supplementation with a FS-HA supplement was associated with an increase in HA concentration in the blood, together with a significant improvement in skin hydration, elasticity, tonicity and wrinkle profile.
The low number of subjects in this study, comprising a panel of females, represents a limitation. Further studies with a larger number of subjects, with modification of hyaluronan dosage and/or duration of treatment period, will likely shed light on the mechanism of action of oral hyaluronan characterized by a wide range of molecular weights.
Disclosures
Acknowledgements: We thank Laboratorio di Analisi Mediche San Giorgio di San Genesio (PV) for the determination of HA serum levels. Financial support: None. Conflicts of interest: AM, EC, ID, MP and FT have no conflicts of interest to disclose.