Epileptic Disorders
MENUClinical experience of abrupt discontinuation of perampanel: a case series Volume 23, numéro 1, February 2021
- Mots-clés : perampanel, discontinuation, experiences, cases
- DOI : 10.1684/epd.2021.1242
- Page(s) : 148-52
- Année de parution : 2021
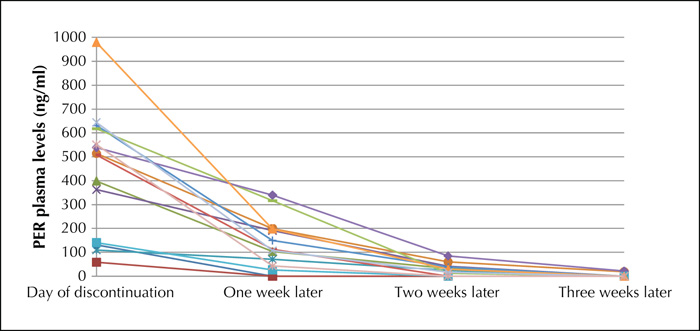
With an elimination half-life of 105 hours, perampanel (PER) allows a once-daily dosing regimen. In pivotal trials, when PER was tapered, it was therefore usually discontinued abruptly. Thus, in our hospital we have always practiced abrupt cessation. In this case series, we investigated how long PER serum concentrations still remain measurable after abrupt discontinuation of PER and whether withdrawal symptoms, such as an increase in seizures or status epilepticus, occur. PER serum levels and the clinical course of 15 adult in-patients were monitored for three weeks based on a retrospective study design following abrupt discontinuation of PER. After one week, PER was still detected in 13 of 15 patients, after two weeks in 10, and after three weeks in three. Neither a severe increase in seizure frequency nor status epilepticus occurred. However, modifications of the concomitant antiseizure drugs were necessary. The abrupt discontinuation of PER leads to a slow decrease in plasma concentration, thus resembling self-evident gradual discontinuation of PER. In some cases, PER may still be measurable and thus clinically active even weeks after its discontinuation. Efficacy and safety of other antiseizure drugs can be estimated appropriately only thereafter.