Epileptic Disorders
MENUClinical experience of abrupt discontinuation of perampanel: a case series Volume 23, numéro 1, February 2021
Antiseizure drugs (ASDs) are usually withdrawn gradually in order to avoid withdrawal symptoms including seizure aggravation or status epilepticus [1, 2]. Abrupt withdrawal of ASDs is only recommended in case of severe adverse events such as allergic reactions, independent of half-life and pharmacological mechanism. Abrupt withdrawal of an ASD with a short half-life (e.g. levetiracetam) can cause an increase in seizure frequency from the first day of withdrawal [3]. Discontinuation of ASDs with a long half-life (e.g. phenobarbital) typically causes an increase in seizure frequency several days up to weeks after discontinuation [4]. Experiences with other ASDs with a long elimination half-life, such as bromides, topiramate or zonisamide, are scarce.
Perampanel (PER) is the first-in-class orally active selective non-competitive antagonist of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors [5] that has been approved as an adjunctive treatment for epilepsies [6, 7]. Due to a long elimination half-life of 105 hours, it can be administered once daily [8]. It has been estimated that abrupt withdrawal of PER does not lead to an increased rate of seizures. A pooled analysis of the three pivotal double-blind Phase III trials, based on questionnaires that were completed at baseline, end of treatment and eight days as well as four weeks after end-of treatment, demonstrated that the abrupt cessation of PER in 832 patients (736 patients during the open label extension phase and 96 patients during the double-blind phase) did not cause behavioural withdrawal symptoms. However, these results were only reported at the time point of eight days after discontinuation and serum levels were not assessed [9].
Since its introduction, we discontinue PER abruptly assuming that, due to the long elimination half-life, self-evident gradual tapering would result. However, we are not aware of any reports in which this has been systematically monitored in clinical practice. Hence, we investigated the clinical course and development of PER serum levels after its abrupt discontinuation in a case series of in-patients at our centre. We were especially interested to determine how long serum levels remain detectable, after which time the influence of PER becomes negligible, and whether severe aggravation of seizures is still possible during or after this time period.
Case study
We retrospectively analysed the clinical course of adult in-patients in whom we had assessed serum levels of their ASDs including PER after the abrupt discontinuation of PER. PER was discontinued either due to a lack of efficacy (six patients) or adverse events (five patients) or both (four patients). Adverse events were depression, psychosis, aggressiveness, dizziness or elevation of liver enzymes. Patient data were included if we had decided to discontinue PER and if PER trough serum concentrations had been measured in our therapeutic drug monitoring (TDM) lab at the time of discontinuation, as well as in weekly intervals thereafter over a period of a at least three weeks. All patients had been on a once-daily PER schedule with oral intake according to bedtime. The TDM methods applied have been reported elsewhere [10]. PER was discontinued abruptly. During the three weeks in which PER levels were measured, other ASDs were added or the dose of prior ASDs was increased. We only used data of in-patients because the use of other, or newly introduced, ASDs replacing PER, as well as the clinical course for at least two weeks following the discontinuation of PER, had been completely documented in the patients’ files. Seizure frequency and potential withdrawal symptoms were assessed by nurses, physicians on daily ward rounds at our centre and continuous video surveillance of the patients.
Fifteen patients were included. The results are summarized in table 1.
All patients suffered from highly active and difficult-to-treat epilepsies. Thirteen patients had focal epilepsy. One patient had generalized epilepsy (Patient 2) and one patient had epilepsy of unknown aetiology (Patient 7). At the time of discontinuation in all 15 patients, the last dose increase in PER had taken place more than two weeks ago, in order to achieve stable plasma steady state conditions.
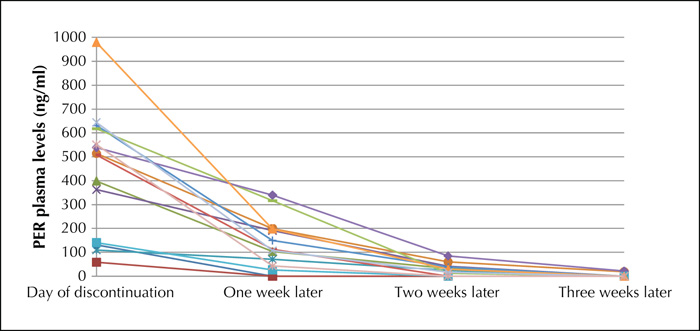
PER dose at discontinuation ranged between 4 and 18 mg (median: 8 mg; mean: 8.9 mg). Plasma levels at discontinuation varied between 59 and 979 ng/mL (median: 514 ng/ml; mean: 460.5 ng/mL). After one week, PER serum levels ranged between 0 ng/mL (two cases) and 396 ng/mL (median: 112 ng/mL; mean: 150.3 ng/mL). After two weeks, PER was no longer detectable in five patients (range: 0 ng/mL-171 ng/mL; median: 24 ng/mL; mean: 34.2 ng/mL). After three weeks, PER was still measurable in three patients. Emergencies or status epilepticus did not occur. However, in all 15 cases, ASD changes were necessary during the three weeks after PER discontinuation due to an unsatisfactory course of the disease.
Mean PER dose was 8 mg (median 6 mg) for the patients with enzyme inducers as comedication (Cases 1, 2, 5, 12, and 13) and 9.4 mg (median 10 mg) for the patients without enzyme inducers. PER plasma concentration at discontinuation was lower for the patients with enzyme inducers as comedication (median: 131 ng/mL; mean: 284 ng/mL) in comparison to the other 10 patients without enzyme inducers as comedication (median: 584 ng/mL; mean: 538 ng/mL). This difference was still measurable one week after PER discontinuation (with enzyme inducers: median=26 ng/mL, mean=59 ng/mL; without enzyme inducers: median=195 ng/mL, mean=195.9 ng/mL). Two weeks (with enzyme inducers: median=0 ng/mL, mean=10.8 ng/mL; without enzyme inducers: median=25 ng/mL, mean=45.9 ng/mL) and three weeks (with enzyme inducers: median=0 ng/mL, mean=0 ng/mL; without enzyme inducers: median=0 ng/mL, mean=12.7 ng/mL) after discontinuation, the PER plasma levels of patients with enzyme inducers approximated those of patients without enzyme inducers.
The individual courses of the PER plasma levels is shown in figure 1.
Discussion
Although based on a relatively small case series, our data show clearly that in each case, we saw a gradual decrease in PER plasma levels. In most cases, PER levels were still detectable even after two weeks, in some instances, even after three weeks. In a previous study, we showed that plasma levels of PER varied widely within a group of highly responsive patients. The lowest PER plasma level in a responder was 52 ng/mL [10]. Although the plasma levels after two and three weeks were low in our present study, we cannot exclude that they might still have reflected some antiseizure activity. One should consider that efficacy and tolerability of the remaining ASDs, independent of PER, can only be evaluated thereafter unequivocally. In our series, the elimination of PER was faster under the influence of potent enzyme-inducing AEDs, such as carbamazepine or phenytoin, similar to other studies [11]. At the same time, PER dose and PER plasma level at discontinuation was lower for the patients with enzyme-inducing comedication. However, the series is too small and the comedications and their changes varied to such an extent that drug-specific effects could not be further addressed. The increased dose of prior medication or the introduced new ASD might also have influenced the PER plasma level.
A significant increase in seizure frequency or status epilepticus was not observed. On the other hand, in each case, we had to modify the antiseizure comedication in order to stabilize the situation. One should not forget that all patients were in-patients due to the highly active and ASD-resistant epilepsy, and that PER was discontinued because of a lack of efficacy in most cases. It is difficult to distinguish between the natural course of the highly active epilepsy and a slight increase in seizures due to discontinuation of PER. Therefore, we focused on significant increase in seizure frequency or status epilepticus, neither of which occurred following abrupt discontinuation of PER.
However, our study protocol represents the usual approach to change AEDs due to continuation of seizures. If one AED is reduced due to lack of efficacy, other AEDs are increased or added. Therefore, the confounding effect of concomitant medication could not be avoided in our series and reflects real-life epileptological practice.
Discontinuation of other AEDs with a long half-life similar to that of phenobarbital can cause withdrawal symptoms and withdrawal seizures [4]. This might be explained by alterations of the hippocampal GABA receptors long after therapy has ended [12] which should not be an issue with PER and its specific mode of action.
Our data show that even the abrupt discontinuation of PER leads to a gradual tapering according to plasma levels. If PER is decreased more gradually, one should consider that this strategy would result in a tapering process of months before the treatment situation can be evaluated without any influence of residual PER.
In our hands, the abrupt discontinuation of PER has appeared to be safe and feasible. We have applied this strategy from the time of introduction of PER in Germany in 2012, without considering an alternative approach. However, our results presented here correspond to closely followed in-patients and should not be translated frivolously to out-patients, even though we use the identical strategy, as caution may be needed in some cases.
To further estimate the risk of increased seizure frequency and status epilepticus after abrupt discontinuation of PER, further studies with a larger number of patients are necessary. It is tempting to speculate that the strategy of abrupt discontinuation might be extended to other ASDs in clinical use with a similar pharmacokinetic profile.
Supplementary data
Summary didactic slides are available on the www.epilepticdisorders.com website.Disclosures
B.J. Steinhoff has received speaker's honoraria from Al-Jazeera, Desitin, Eisai, GW Pharmaceuticals, Hikma, Novartis, Sandoz, and UCB and has served as a paid consultant for Arvelle, Bial, B. Braun, Desitin, Eisai, GW Pharmaceuticals, and UCB.
A.M. Staack has received speaker's honoraria from Eisai, Desitin, and UCB.
C. Kurth has received speaker's honoraria from Desitin, Eisai and UCB.
M. Blickhan, T. Intravooth, R. Kornmeier, P. Mahn, M. Schneider, J. Stockinger, M. Bacher have no conflicts of interest to declare.