Epileptic Disorders
MENULesion-negative anterior cingulate epilepsy (WITH VIDEO) Volume 17, numéro 2, June 2015
Anterior cingulate epilepsy (ACE), a rare entity (Garzon and Lüders, 2008; Alkawadri et al., 2013), presents particular challenges in focus localization during pre-operative epilepsy surgery work-up (Garzon and Lüders, 2008; Alkawadri et al., 2013). The anatomical location of the cingulate gyrus (CG), medial and distant from the cerebral surface, makes localization of the ictal onset zone difficult. The phenomenon of secondary bilateral synchrony (SBS) increases the lateralization difficulty (Cukiert et al., 1991; Iwasaki et al., 2011). Extensive connectivity demonstrated between homotopic cingulate and mesial frontal regions across the corpus callosum in mammals (Marcus and Watson, 1968; Musgrave and Gloor, 1980; Umeoka et al., 2010; Iwasaki et al., 2011) may provide the basis for the lateralization difficulties encountered in humans. Localization and lateralization challenges are compounded in MRI-negative patients (Cukiert et al., 1999; McGonigal et al., 2008). Although semiological descriptions of ACE exist (Bancaud and Talairach, 1992; Alkawadri et al., 2013), recent reports suggest semiological heterogeneity between different frontal regions and even within the subgroup of ACE (Alkawadri et al., 2013; Bonini et al., 2014). Herein, we describe an MRI-negative ACE case in which semiology, surface EEG, and imaging were non-contributory, but the presence of extremely focal interictal and ictal high-frequency discharges and electrical stimulation of the putative ictal onset zone resulted in successful localization, lateralization, and resection of the epileptogenic zone. We posit that in ACE cases in which semiology, conventional scalp EEG and imaging are unhelpful, additional scrutiny of the EEG for high-frequency oscillations (HFOs) and the use of 50-Hz electrical stimulation for habitual seizure induction can be of critical benefit.
Case study
A 30-year-old, right-handed male with medically intractable focal epilepsy of 22 years duration was referred for pre-surgical evaluation after multiple attempts at medical control. At evaluation, he was on levetiracetam (500-500-500 mg), topiramate (50-50-100 mg) and lamotrigine (400-200-500 mg). Gestational, birth and development histories were normal and he had no history of epilepsy risk factors. He had 1-2 nocturnal seizures per month, characterized by a non-specific cephalic aura, followed by hypermotor seizures with pedalling movements in both legs and prominent gestural automatisms. Awareness was altered for less than a minute with each seizure. Neurological and neuropsychological examinations were normal. Epilepsy protocol 3T MRI was consistently normal, as was FDG-PET.
Semiology
Several seizures were captured with EEG onset, the distribution of which was the same as that for interictal discharges. Seizure semiology was characterized by a cephalic aura, followed 10-15 seconds later by prominent, repetitive, high-amplitude, proximal lower limb movement, rocking, agitation, tachycardia, pupillary dilation, and a fearful facial expression. Approximately 25 seconds after seizure onset, he had prolonged ululating palilalia of 15-18 seconds duration, accompanied by upward right conjugate gaze deviation (see video sequence). A decision was therefore made to evaluate the patient with stereotactic EEG (SEEG) in both frontal lobes with a putative epileptogenic zone in the mesial frontal lobe, anterior to Brodmann area 6. Adjacent dorsolateral premotor and prefrontal cortices were also covered.
Electrophysiology
1) Interictal surface EEG revealed generalized spikes, polyspikes, and runs of spike-wave complexes, with varying left or right fronto-central emphasis (maximum at Cz>FZ on 10-20 electrode montages) (figure 1). EEG source imaging (Compumedics Neuroscan Curry 6.0.1; electrode locations digitized by Polhemus FastTrak electromagnetic 3D digitizer; MRI preprocessing with MPRAGE acquisition protocol) consistently localized the epileptiform discharges to deep-seated midline structures. Symmetric bifrontal SEEG evaluation with electrode coverage revealed fronto-central spikes bilaterally, similar to scalp discharges with no clear interictal or ictal lateralization. However, maximal activity was seen bilaterally in the ventro-mesial frontal, anterior peri-cingulate regions. A decision was therefore made to carry out a second SEEG evaluation, concentrating on the anterior cingulate gyrus and adjacent brain regions not targeted by the first evaluation.
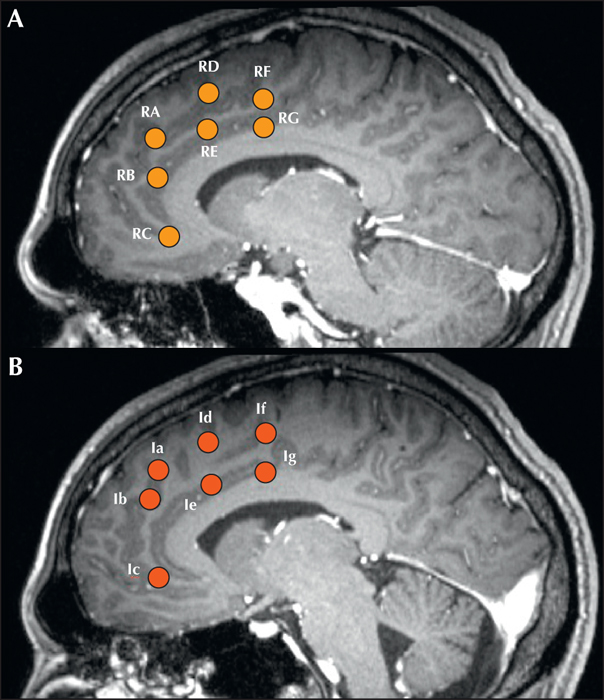
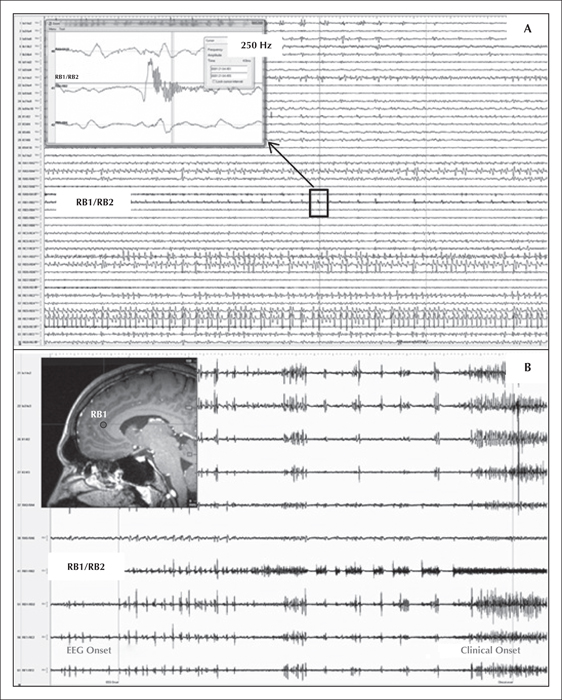
2) Intracranial EEG. SEEG electrodes were placed orthogonally with mesial contacts in the mesial frontal walls of both frontal lobes, including both anterior cingulate/pericingulate regions (figure 2). Antiepileptic medication was reduced and stopped in a phased manner over five days. Frequent interictal bifrontal discharges, similar to the previous evaluation were seen in the anterior cingulate and adjacent cortex, again without clear lateralization, although some discharges had maximum amplitude in the left mesial frontal contacts (le2/le3/lf1/lf2; figure 1). However, using high-frequency analysis settings (high-cut filter: 600 Hz; high sensitivity setting; time constant: 0.0001 ms) a distinct 250-Hz high-frequency oscillation pattern was seen over-riding the sharp-wave population. These HFO were seen exclusively at RB1/RB2 electrodes in the right anterior cingulate region, in the inferior bank of the cingulate sulcus anterior to the genu of the corpus callosum (figure 3A). Besides, a 250-Hz fast-frequency ictal EEG onset was seen arising from the same contacts, in all habitual seizures, >20 seconds before clinical onset. Electrical stimulation was then carried out with particular emphasis on the more active electrodes on either side. Stimulation of 50 Hz (0.2-ms pulse width, 2-mAmp intensity) of electrode contacts RB1/RB2 produced the patient's habitual seizures in a consistently reproducible manner over two days of repeated testing (figure 3B). Current intensity of up to 20 mAmp for 10 seconds failed to produce seizures or discharges in other electrodes.
Anatomy
A consensus decision was then made to resect the right anterior cingulate region. At operation, the adjacent pericingulate areas involved in the interictal discharges were also removed to achieve a better outcome. A tailored resection of the right mesial frontal lobe was performed with the posterior resection margin at the anterior border of the supplementary motor area, inferior margin at the corpus callosum, and extending anteriorly and inferiorly to the floor of the anterior fossa, to include ictal, as well as the prominently discharging, interictal, contacts (figure 4). Neuropathology showed focal cortical dysplasia (FCD) type 1b (Blumcke and Muhlebner, 2011) (figure 5). Forty-eight hours of prolonged post-operative EEG showed absence of any interictal discharges. The patient has been seizure-free at one year follow-up.
Discussion
This case emphasizes several practical difficulties with localization and lateralization of the epileptogenic zone to the ACG in MRI-negative cases and highlights the contributory roles of clinical semiology and electrophysiology (SEEG evaluation, high-frequency discharges in invasive EEG, and electrical stimulation for habitual seizure induction) in determining the anatomical location of the epileptogenic zone.
In epilepsy surgery practice, the frontal lobe is the most commonly operated brain region after the temporal lobe. However, electroclinical frontal lobe epilepsy features are less well characterized than temporal lobe epilepsy (Bancaud and Talairach, 1992; Bonini et al., 2014). Several authors have differentiated between seizures arising from various regions of the frontal lobes (Bancaud and Talairach, 1992; McGonigal and Chauvel, 2004; Garzon and Lüders, 2008; Alkawadri et al., 2013; Bonini et al., 2014), but the anterior cingulate gyrus (ACG), appears to show significant heterogeneity in its manifestations (Alkawadri et al., 2013). These semiological and other features of ACE reported in literature are detailed in table 1.
Ictal semiology in relation to ACE is relatively varied. The most frequent seizure types reported to originate from the ACG are hypermotor, gelastic, complex motor, and bilateral asymmetric tonic seizures (table 1). Bancaud's description of ACE consists of emotional symptoms, intense fright with facial expressions of fear, shouting, complex motor manifestations sometimes with aggressive verbalization, autonomic symptoms, and alteration of consciousness (Bancaud and Talairach, 1992). This was found in 6 of 10 ACE patients in a recent series (Alkawadri et al., 2013) and labelled as “typical” ACE symptomatology. The remainder was classed with “atypical” seizures and characterized by tonic rather than hypermotor features, suggesting heterogeneity in ACE semiology. Moreover, “typical” ACE manifestations can also occur in other prefrontal regions (Chang et al., 1991; Munari et al., 1995). Our patient's presentation with hypermotor seizures, fearful facial expression, and autonomic symptoms (pupillary dilation and tachycardia) is consistent with “typical” ACE. Vocalizations are consistent with both typical and atypical ACE phenotypes reported by Alkawadri et al. (2013). Autonomic and “neurovegetative” features, such as in our patient, are well described in ACE, as well as other brain regions (orbitofrontal, operculo-insular).
Non-invasive neurophysiological studies in patients with ACE provide only partial or inconclusive data because of the anatomical location of the CG (medial and distant form the cerebral surface). This is reflected in the secondary bilateral synchrony phenomenon, seen in our case, defined as apparent generalized epileptiform discharges triggered by a focus. It can pose significant lateralization/localization problems and often necessitates invasive evaluations (table 1). It is well described in frontal lobe epilepsies, especially in the mesial region and is a frequent lateralization conundrum (Marcus and Watson, 1968; Musgrave and Gloor, 1980; Cukiert et al., 1991; Cukiert et al., 1999; Lacruz et al., 2007; Umeoka et al., 2010; Iwasaki et al., 2011). Extensive connectivity between homotopic regions of both frontal lobes is a likely explanation (Lacruz et al., 2007; Iwasaki et al., 2011). Primate studies have shown that the mesial frontal regions are very heavily supplied by reciprocal callosal connections (Karol and Pandya, 1971) although human evidence has been mostly indirectly ascertained. Transcallosal inter-hemispheric conduction, using the relationship between axonal diameter, conduction velocity, and inter-hemispheric transfer, is estimated to be 1.5 to 24.9 ms (Aboitiz et al., 1992) in humans. This is supported in one study of single-pulse 1-ms electrical stimulation of 23 medial frontal lobes assessed for epilepsy surgery which showed early responses (median: 30 ms [15-45 ms]) in the contralateral mesial frontal lobe in 14 (61%) (Lacruz et al., 2007), best explained by corpus callosal connections (Buser et al., 1992). EEG source imaging can contribute to localization (Brodbeck et al., 2011) although in our case, the deep source was consistently unlateralized and in the midline. Indeed, some of the bifrontal interictal discharges, misleadingly, were maximum in the left frontal lobe.
HFOs (>80 Hz) are seen primarily in the epileptogenic zone (Jacobs et al., 2010a, 2010b; Wu et al., 2014). Furthermore, a negative correlation between HFO rates and stimulation response thresholds has been reported (Jacobs et al., 2010b). Our case exhibited interictal 250-Hz HFOs in the ACG and a consistent similarly fast-frequency ictal onset discharge from the same region. Consistent with the literature, low-current intensity 50-Hz stimulation of the fast-frequency discharge interictal and ictal contacts consistently induced the patient's habitual seizures. Electrical stimulation of small, putatively epileptic cortical areas may provide data complementing that provided by spontaneous seizure recordings (Kahane, 2004). The area delineated by HFOs and electrical stimulation in our patient was therefore likely to be part of the epileptogenic zone.
The absence of an MRI lesion in this case is not surprising given that the pathology was subsequently revealed to be FCD type 1b. However, the absence of an MRI lesion posed significant difficulty in localization of the ictal onset zone, which in the absence of SEEG would not have been feasible. MRI-negative ACE is extremely rare and only a handful of cases exist in the literature (table 1). This case demonstrates the potential rewards of keeping this entity in mind in MRI-negative prefrontal lobe epilepsy and in particular, comprehensively targeting the ACG with SEEG electrodes (along with other candidate frontal regions), which allows for the scrutiny of HFOs, as well as provocative electrical stimulation in deep cortical structures, such as the ACG.
One limitation of this study is the relatively extensive medial frontal resection that was performed in spite of the presurgical evaluation pointing to a very focal ACE, in keeping with some of the cases detailed in table 1. However, the presurgical evaluation in this case strongly suggests that the epileptogenic zone was indeed limited to the ACG.
Conclusion
Our patient's evaluation and outcome suggests that although rare, MRI-negative ACE is an entity that exists and can be identified through careful anatomo-electro-clinical analysis. Patients with frontal lobe seizure semiology (hypermotor or prominent gestural automatisms, fearful expression, autonomic signs, and palilalic vocalizations), exhibiting non-lateralizable bifrontal discharges and non-contributory structural, functional or source imaging, may yet have surgically amenable ACE. Careful SEEG evaluation for interictal and ictal HFOs with additional cortical stimulation for habitual seizure induction provides the best opportunity for an accurate localization of the epileptogenic zone.
Acknowledgements and disclosures
None of the authors have any conflict of interest to disclose.