Hématologie
MENUIndications and procedure for CAR T-cell infusion Article à paraître
Illustrations
Tableaux
Chimeric antigen receptor T (CAR-T) cells have recently been shown to be effective in childhood, adolescent and young adult B-acute lymphoblastic leukaemia (B-ALL) and in relapsed or refractory adult diffuse large B-cell lymphoma (R/R DLBCL). In the ELIANA trial, which included patients with B-ALL under the age of 25, tisagenlecleucel (tisa-cel) achieved an overall response rate (ORR) at three months of 81%, an event-free survival (EFS) at 12 months of 50% and an overall survival (OS) at 12 months of 76%. The rate of cytokinin release syndrome (CRS) was 77% (of which 46% were Grade 3–4) and the rate of neurological events was 40% (of which 13% were Grade 3-4) [1].
In DLBCL, primary mediastinal B-cell lymphoma (PMBCL) and transformed follicular lymphoma (TFL), three main clinical trials are highlighted:
- –ZUMA-1: axicabtagene ciloleucel (axi-cel) (Yescarta®) [2],
- –JULIET: tisa-cel (Kymriah®) [3],
- –TRANSCEND-NHL-001: maraleucel lisocabtagen [4].
In these trials, the ORR rate is 52%–83% and the complete response rate (CRR) rate is 40%–58%. The median progression-free survival (PFS) and median OS are 2.9–6.8 months and 12–25.8 months, respectively. The rates of CRS and immune effector cell-associated neurotoxicity syndrome (ICANS), previously called CAR-related encephalopathy syndrome (CRES), of Grade >3 are 2%–22% and 10%–32%, respectively [2–4].
From a regulatory perspective:
- –tisagenlecleucel has marketing authorisation:
- –axicabtagene ciloleucel (Yescarta®) has been granted marketing authorisation:
- •for third line or higher treatment of R/R DLBCL [7] and R/R TFL - for third line or higher treatment of R/R PMBCL.
In 2019, the two leading commercial CAR-Ts, axi-cel and tisa-cel, were awarded the Prix Galien in the “innovative therapeutic medicine” category.
Criteria for patient eligibility
When a patient's referring haematologist is considering a CAR-T indication for a given patient, they must first assess the patient's eligibility. This assessment takes place between the haematologist in charge of the patient, whether they are from another centre or the same department, and the haematologist from the CAR-T accredited centre. The final decision will be explained to the patient. Contraindications to axi-cel and tisa-cel are hypersensitivity to the active substance or to one of its excipients, and contraindications to lymphodepletion chemotherapy [8,9].
The eligibility criteria we use are largely derived from those used in clinical trials. There are more or less significant differences between the eligibility criteria of the different clinical trials, those used by our centre (haemato-oncology department, Saint-Louis Hospital), and those recommended by the European Society for Blood and Marrow Transplantation (EBMT) (Table 1). Regarding age, we chose the arbitrary threshold of 80 years, but physiological age took precedence over actual age. Although there are some differences in the time to complete remission, a recent history of neoplasia is one criterion for ineligibility for CAR-T. Even a recent history of carcinoma in situ or non-melanoma skin cancer are tolerated. There is little or no experience of using CAR-Ts in patients with replicative HIV, hepatitis B or C (HBV/C) viral infection, or central nervous system (CNS) involvement and, therefore, CAR-Ts should be avoided in these patients. The existence of an active or severe neurological co-morbidity (e.g. advanced Parkinson disease, active multiple sclerosis or amyotrophic lateral sclerosis complicated by disability, advanced neurodegenerative disease) is considered to be a criterion for non-eligibility by our centre. Although the existence of an autoimmune disease is not an exclusion criterion in the major CAR-T clinical trials, our centre, as well as the EBMT, considers active and uncontrolled autoimmune disease to be a criterion for non-eligibility for CAR-T. Organ damage during these diseases can make the use of CAR-Ts difficult, as can the use of immunosuppressive treatments that can alter the function and persistence of CAR-T cells in vivo. In addition, there is a theoretical risk of severe CRS or ICANS in the inflammatory context of uncontrolled autoimmune disease. In addition, while clinical trials and our centre consider that creatinine clearance of more than 60 mL/min is necessary, the EBMT prefers to set a less restrictive threshold of 30 mL/min, although a precautionary statement should be made about use in cases of clearance between 30 mL/min and 60 mL/min. In our experience in the haemato-oncology department of the Saint-Louis Hospital, only one-third of patients are considered to be eligible for CAR-T treatment. One-third are rapidly excluded because they do not fit the indication, and one-third are judged ineligible, the two main causes being:
- –the overly progressive nature of the lymphoma (compressive mass, performance status [PS] > 2, rapid elevation of lactate dehydrogenases [LDH]),
- –the existence of significant comorbidities [10].
These co-morbidities are most often organ failure (mostly heart and kidney failure), advanced neurological diseases, active autoimmune diseases, HIV, HBV and/or HCV viral infections, and being over the age of 80. A patient receiving CAR-T cells should be able to withstand the equivalent of septic shock and its treatment (vascular filling, use of catecholamines), as CRS may share its clinical presentation and severity. Achieving CR after bridging chemotherapy is currently a criterion for ineligibility in our centre, as the lack of a target would not allow for CAR-T expansion. For example, in ZUMA-1 [11], patients in CR at the end of bridging chemotherapy were excluded, and to date there is no demonstration of the benefit of CAR-Ts in the CR situation in lymphomas. Furthermore, concerning a possible treatment of minimal residual disease (MRD) by CAR-T, this is currently not routinely evaluated. It should be noted that previous treatment with blinatumomab (scFv CD3xCD20) is not a contraindication to the use of anti-CD19 [12].
Care pathway: from inclusion to reinjection of chimeric antigen receptor T cells
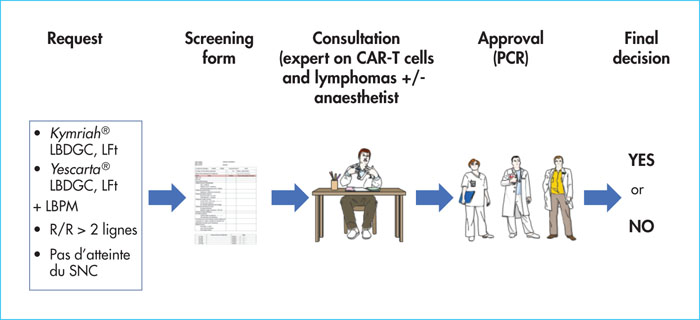
Each patient for whom CAR-T treatment is proposed follows a centre-specific procedure. There are organisational differences between the French CAR-T certified centres concerning, for example, the location for apheresis (French Blood Establishment [EFS] or hospital apheresis facility) or the location for reinjection (allograft department or haematology department). In any case, the patient selection procedure is broadly similar (Figure 1).
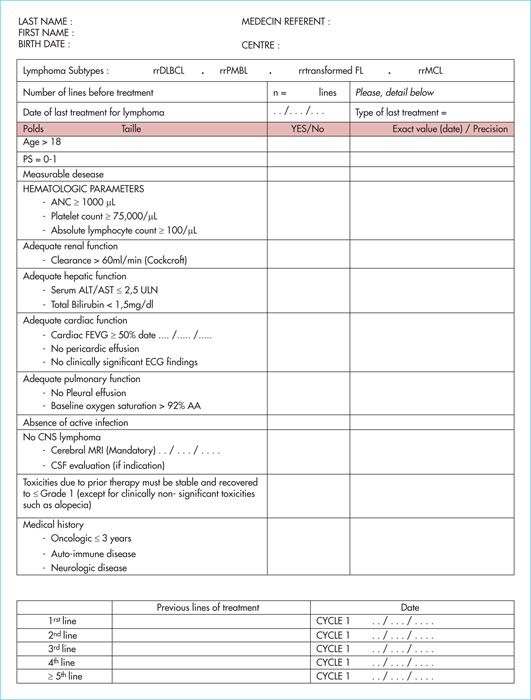
In our experience, a form to check the patient's eligibility criteria is filled in either by us, when the patient comes from our centre (haemato-oncology department, Saint-Louis Hospital, Paris), or by the patient's referring haematologist and sent to one of the haematologists of our CAR-T-approved centre, for patients coming from another centre (Figure 2). This form is accompanied by a detailed history of the patient's haematological disease, history and comorbidities, the pathological report and the reports of the last scans and/or PET scans. After validation of these criteria by one of the haematologists at our centre, the patient will be seen in consultation by a haematologist in charge of CAR-T reinjection. The time between the validation of the criteria by the CAR-T expert and the specific consultation must be rapid, if possible less than three to five days, given the particularly aggressive nature and rapid evolution of these lymphomas, which are often refractory to all chemotherapy and which will make bridging chemotherapy difficult. In the case of significant comorbidities and/or age > 70 years, the patient may also be examined concomitantly by an anaesthesiologist who will judge the patient's ability to endure a stay in the intensive care unit. During the consultation with the CAR-T expert haematologist, the clinical and biological eligibility and exclusion criteria will be reviewed again. The final decision will be approved in a multidisciplinary consultation meeting after reviewing the imaging (CT and PET) with radiologists and nuclear medicine physicians, to decide on the tumour volume and the sites of damage, particularly extra-ganglionic sites (cardiac, cerebral, etc.). As soon as CAR-T treatment is approved, cytapheresis, to collect T cells, is arranged according to the available transport and manufacturing dates provided by the pharmaceutical companies. The speed and reactivity of the teams (haematologists from the patient's centre of origin and CAR-T centre, the cytapheresis unit, cell therapy unit, hospital pharmacy, CAR-T coordination nurses and private companies) is fundamental when faced with rapidly progressive haemopathies. The average time between cytapheresis and reinjection in France is currently 30 to 45 days [10,13]. The cytapheresis product must be cryopreserved on site for certain cell products (tisa-cel).
As soon as cytapheresis is performed, and if the patient so requires, bridging chemotherapy is administered, not to put the disease in remission before reinjection, which is most often illusory given the refractory nature of these lymphomas, but to stabilise it and maintain the patient in a sufficiently good general condition to carry out the whole procedure. This chemotherapy, which is necessary in 93% of cases [13], may be more or less intensive: initial data suggest that low-dose protocols (e.g. rituximab + dexamethasone, lenalidomide) are preferable in order to limit infectious complications or subsequent CAR-T toxicity. We have shown that the intensity of this bridging chemotherapy did not influence the efficacy of CAR-T [14]. There is no consensus on this bridging chemotherapy and practices often vary within the same centre. The choice is made on a case-by-case basis and depends on factors related to the disease (tumour mass, location, proliferation kinetics, response to different previous lines, immunohistochemical markers) and factors related to the patient (general condition, comorbidities, tolerance of previous treatments) [12]. It can be performed by the CAR-T expert centre or at the centre of origin for the convenience of the patient. It should only be started after cytapheresis, so as not to reduce the quality of the cytapheresis product. In the case of lymphoma, a PET scan is performed before and after this chemotherapy. Upon receipt of the CAR-Ts, the patient is hospitalised to begin lymphodepletion prior to reinjection of the CAR-Ts. It is necessary to ensure that the cells have been received before starting this lymphodepletion. Lymphodepletion is a short course of chemotherapy (three to five days before CAR-T injection) aimed at creating a favourable environment for CAR-T in vivo. Different treatment regimens and molecules were used in the initial trials. The most commonly prescribed combination, offering optimal expansion and persistence of CAR-Ts [15], is the combination of fludarabine and cyclophosphamide (fludarabine 25 mg/m2 + cyclophosphamide 250 mg/m2 for tisa-cel and 30 mg/m2 + 500 mg/m2 for axi-cel). Active infection should be ruled out before lymphodepletion is started (see below).
The CAR-T cells are sent to the department after telephone contact between the hospital pharmacy and the haematologist responsible for reinjecting the CAR-Ts, in order to ensure that the medical and paramedical team is ready to inject the cells upon reception. The injection is carried out via a central venous line, preceded by premedication with paracetamol and antihistamines. Corticosteroids should be avoided in order to prevent alteration of the CAR-Ts. The duration of the injection should be less than 30 minutes and immediate tolerance is generally excellent. Any active infection should be ruled out before starting the injection (see below).
Practical procedures for stopping antineoplastic and immunosuppressive drugs
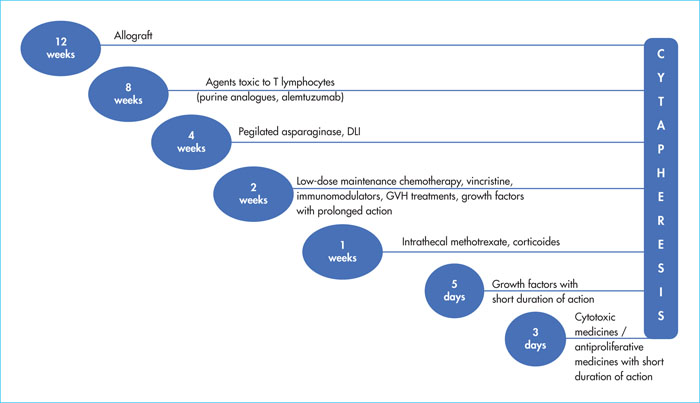
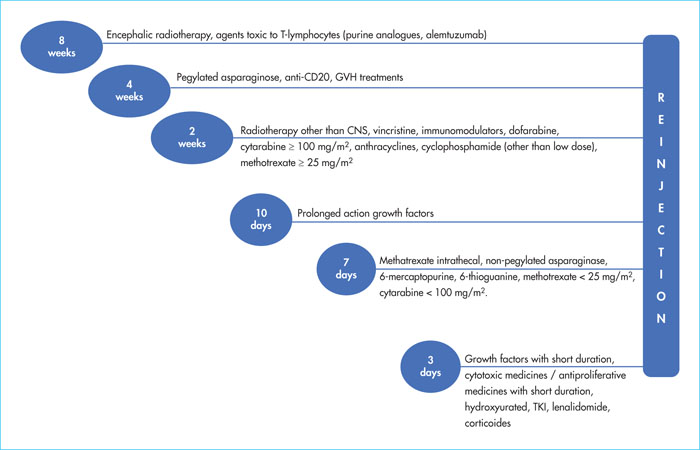
One of the most common questions in our discussions with patients referred to haematologists is the minimum time required between the cessation of the various targeted/chemotherapy/immunosuppressive treatments and cytapheresis or CAR-T reinjection. Figures 3 and 4 show, in a very practical way, the time required for each type of treatment, before cytapheresis and before reinjection of CAR-T cells. These times limit possible negative effects on efficacy or toxicity of CAR-T and/or lymphodepletion [12,16].
Care pathway: post-injection hospitalisation and medium- and long-term monitoring
After the reinjection of CAR-T cells, patients are hospitalised for 10 to 14 days (pharmaceutical and EBMT guidelines) [12]. The occurrence of CRS or ICANS complications can of course prolong this period. Patients most often have aplasia and the three main risks are: CRS, ICANS and infectious complications. Patients may require transfusions of platelet and red blood cell concentrates. A physician should be seen daily or even several times a day. In the haemato-oncology department of the Saint Louis Hospital, a daily chart is filled with the day's vital and important clinical events. The patient also writes a line of text every day, as the occurrence of dysgraphia is an early sign of ICANS.
From discharge to D28, it is recommended that patients remain within close proximity (< 60 minutes) of a centre or hospital competent in the management of post-injection complications [12]. Indeed, complications can occur after discharge (medium-term complications, i.e. from D28 to D100): primarily ICANS, but also delayed CRS, infectious complications, and late cytopenias. Delayed CRSs (between D14 and D21) are mainly seen in chronic lymphocytic leukaemia (CLL). In the haemato-oncology department of the Saint-Louis hospital, patients have a daily consultation, either physically or by telephone, with a coordinating doctor or nurse from D14 to D21. Patient education is fundamental here; patients should be aware of the warning signs that may suggest CRS, ICANS or infection, and should promptly contact the CAR-T centre if any of these signs appear. Furthermore, driving is contraindicated for eight weeks following the injection due to the risk of ICANS (impaired alertness, confusion, epilepsy) [12]. In high-grade B-cell lymphoma, PET scans are routinely performed at D30 and D90 to assess response to treatment. They can be performed earlier if early progression is suspected.
Long-term follow-up (after D100 post-injection of CAR-T) is essential, although we still have little experience of this given the recent introduction of the treatment into the therapeutic armamentarium. Follow-up should focus on detecting relapse, looking for late toxicity, including infection, secondary cancers, metabolic and cardiovascular sequelae and psychosocial sequelae. It should also focus on assessing patients’ quality of life [12].
Management of cytokine release syndrome
CRS is the most common complication of CAR-T therapy. It is caused, as the name suggests, by cytokine storm (notably interleukins 6 and 1 [IL-6, IL-1], and interferon γ [IFNγ]). The cells responsible for this release are still poorly identified but could be CAR-T, monocyte-macrophages or even the tumour cells themselves. Indeed, preclinical studies have recently shown the role of monocyte-macrophages in the release of pro-inflammatory cytokines and the occurrence of CRS and ICANS [17]. The inhibition of granulocyte-macrophage colony-stimulating factor (GM-CSF), by gene deletion or use of monoclonal antibodies, promotes the effector functions of CAR-T cells, while decreasing CRS and ICANS [18]. For example, a Phase I/II trial will soon begin to evaluate the use of lenzilumab, an anti-GM-CSF monoclonal antibody, in patients treated with axi-cel (ZUMA-19, NCT04314843).
Risk factors for CRS include large tumour volume, lymphodepletion with fludarabine and cyclophosphamide, thrombocytopenia prior to lymphodepletion, large doses of CAR-T administered, and the type of CAR construct [19]. CRP and ferritin levels would be useful in predicting and monitoring the onset and progression of CRS and ICANS. The advantage of these assays is that they can be performed routinely, unlike the assays of serum levels of various cytokines, such as IL-6 [12].
The severity of CRS varies from a simple fever to a condition resembling septic shock, which leads to the patient being admitted to the intensive care unit. The various clinical signs that may suggest CRS are fever (which is constant and often the first sign), chills, dyspnoea, low blood pressure and tachycardia. The diagnosis of CRS is based on a number of features, primarily clinical. It is often not easy to determine the origin of the fever in these patients, who often have aplasia and for whom a broad-spectrum probabilistic antibiotic IV therapy will be started. The prognosis may be life-threatening in the case of multiple organ failure. CRS can also be the cause of macrophage activation syndrome (MAS). The median time to onset of symptoms is two days after reinjection of CAR-Ts (1–14 days) and the median duration of the CRS is seven days (1–10 days). The most severe CRS (Grades 3–4) occurs most often within the first three days after the injection.
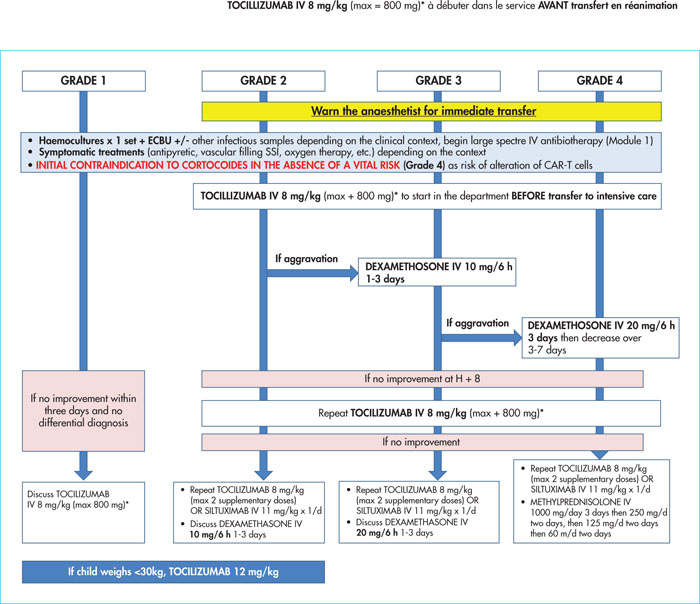
CRS is a therapeutic emergency; a delay in management can lead to the rapid death of a patient. The management procedure must be known by the medical and paramedical teams in haematology and intensive care. The procedure depends on the severity of the CRS and different scales have been proposed to quantify severity. Efforts have been made to standardise the management of CRS and to facilitate the comparison of results between studies. The publication by Lee et al. is now finally gaining acceptance (Table 2). This scale is based on the existence of fever, arterial hypotension (with or without the use of vasopressive amines) and/or hypoxia (using modalities and an appropriate level of oxygen therapy) [20]. Also, with a view to harmonising practices, a recent publication by Yakoub-Agha et al. proposes a therapeutic algorithm for the management of CRS according to its grade of severity (Figure 5). In the case of CRS, patients must be monitored closely, as the situation can deteriorate very quickly. From Grade 2 onwards, anaesthetists should be contacted for emergency transfer, and tocilizumab, a monoclonal antibody to the IL-6 receptor (IL-6 R), should be started immediately. High-dose corticosteroid therapy (dexamethasone) should be reserved for CRS not responding to tocilizumab, due to possible alteration of CAR-Ts by corticosteroids [12].
Management of central neurological toxicity
ICANS is the second most common complication of CAR-T. Its pathophysiology is much less known than that of CRS. IL-1 seems to play an important role in its genesis. Indeed, in mouse models, inhibition of IL-6 R has no effect on neurological toxicity, unlike inhibition of IL-1 R. This toxicity is similar to that observed during treatment with blinatumomab. It should be noted, however, that there is usually no neurological toxicity without prior CRS, demonstrating a commonality in the origin of these two toxicities.
Risk factors for ICANS include ALL, CRS, brain involvement during the haematological disease, large tumour volume, high dose of CAR-T administered, and pre-existing neurological comorbidities [21].
The severity of ICANS, like CRS, is highly variable, ranging from simple attention deficit to severe coma, status epilepticus and cerebral oedema. The positive diagnosis is based on a range of features, especially clinical and contextual. The clinical signs may include tremors, phasic disorders, dysgraphia, visual disorders due to papilloedema, seizures, attention disorders, vigilance disorders, coma, confusional syndrome, stupor, agitation, hallucinations and sensory-motor deficits. Dysgraphia is a prodromal sign, which is why handwriting tests are undertaken from D1 post-injection. The presence of isolated headaches is not sufficient to make a diagnosis of ICANS. An increase in intracranial pressure may be observed. An emergency fundoscopy should be requested to look for papilloedema. ICANS most commonly occurs at the end of, or following resolution of CRS, but onset may also be delayed to three to four weeks after CAR-T injection (median time to onset of symptoms: six days [1–34 days]). The median duration of these symptoms is five days (1–21 days).
A consultation with a neurologist and a neuropsychologist is essential before reinjecting CAR-T cells. The neurologist looks for any abnormalities in the neurological examination that may indicate the existence of CNS damage caused by the disease or a neurological comorbidity, potentially contraindicating the use of CAR-T. The neuropsychologist, a clinical psychologist interested in the relationship between the brain and psychological functioning, uses standardised scales to look for cognitive or behavioural disorders that may indicate CNS damage. Brain MRI is mandatory in the pre-treatment work-up; primary brain lymphoma (even if it is a DLBCL) is not currently an indication for CAR-T, and the existence of brain involvement by the haemopathy is considered by most centres as a contraindication, as the risk of ICANS is likely to be increased in this latter situation. Neurological examinations should be carried out on a daily basis during hospitalisation, or even several times a day in the event of suspected ICANS, as the symptoms can rapidly evolve within a few hours. Primary prophylaxis with anticonvulsants (e.g. levetiracetam 500 mg/d from D1 to D30) may be indicated by the neurologist if there are risk factors for ICANS (history of epilepsy, history of CNS disease).
As with CRS, several scales exist to measure the severity of ICANS. A recent effort to harmonise practices has been made and the recommendations of Lee et al. are becoming more widely used. The ICANS grade is established in this scale using five items: the immune effector cell-associated encephalopathy (ICE) score, epilepsy, motor skills, level of consciousness and cerebral oedema. The ICANS grade depends on the most severe item (Table 3). The ICE score, which is one of the five items used to establish the ICANS grade, is calculated using a practical and easy-to-perform neurological examination and is based on five points (Table 4) [20]. Brain MRI, lumbar puncture, fundoscopy and EEG should be discussed as a matter of urgency in the presence of any suspicion of ICANS, and these tests should be repeated until the symptoms are resolved.
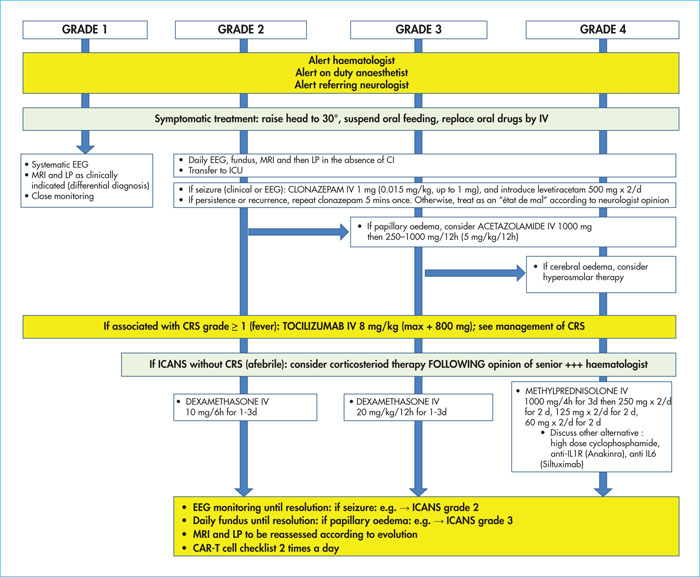
ICANS is also a therapeutic emergency. The course can be very rapid and potentially fatal if not managed optimally. Treatment is guided by the severity of the syndrome, hence the importance of classifying ICANS according to grade. Several treatment algorithms have been proposed, the most recent (Figure 6) of which is based on the EBMT and JACIE recommendations. The on-duty anaesthetist and the neurologist must be systematically called, and transfer to the intensive care unit must be carried out as a matter of urgency from Grade 2. In the absence of association with CRS, treatment is based on dexamethasone as first-line therapy, starting at Grade 2. Efficacy is generally very rapid, and care must be taken not to reduce corticosteroid therapy too quickly, given the possible risk of recurrence. However, if combined with a CRS, tocilizumab should be offered as first-line therapy. Symptomatic treatments are important here: tilting the head to 30° in case of suspected cerebral oedema, relaying IV drugs, clonazepam in case of epileptic seizures, acetazolamide in case of papilloedema, and hyperosmolar hydration in case of cerebral oedema [12]. The impact of anti-IL-6 R and corticosteroid use on CAR-T efficacy has been evaluated in several studies and no significant difference in response has been observed between the different patient groups [22].
The American Society for Transplantation and Cellular Therapy (ASTCT) has created a mobile application which can be downloaded free of charge, to quickly determine the CRS and ICANS grade. However, it is important to remember that determining the grade of a toxicity is not a substitute for appropriate management of a patient's clinical condition.
Cytopenias
As a rule, pancytopenia occurs after lymphodepletion followed by CAR-T reinjection. There is usually transient aplasia, and the patient may require transfusions of platelet concentrates and/or erythrocytes. However, cytopenias may persist later (beyond 30 days after injection). Approximately one-third of patients have prolonged neutropenia beyond 30 days post-injection and 20% have neutropenia lasting more than 90 days. In particular, a biphasic course of neutropenia and thrombocytopenia has been described: an initial phase of early cytopenias, followed by a transient improvement, and then a second phase of late, sometimes severe cytopenia. In the case of neutropenia, an Israeli team found a correlation between serum levels of stromal derived factor 1 (SDF-1; CXCL-12) and the absolute value of neutrophils beyond D28 [23]. In the literature, late neutropenia has been correlated with B cell reconstitution after rituximab treatment, with a causal role for SDF-1 [24]. Thus, this team proposes a model in which early neutropenia is related to lymphodepletion and CRS, while some late neutropenia is partly due to consumption of SDF-1 by post-CAR-T [23]. Indeed, SDF-1 is a chemokine with a role in both early B lymphopoiesis, neutrophil migration and haematopoietic stem cell survival/migration. However, our observations show that B lymphodepletion persists while neutropenia also persists. Other avenues remain to be explored.
The use of G-CSF is debated. Indeed, it could favour the occurrence or increase the severity of CRS or ICANS. The EMBT recommendations are to discuss the use of G-CSF in cases of prolonged neutropenia from D14 post CAR-T [12].
Hypogammaglobulinaemia
As a consequence of CD19 targeting by CAR-Ts currently on the market, hypogammaglobulinaemia is related to B-cell aplasia. The immunosuppression of patients and the risk of infection are partly encouraged by hypogammaglobulinaemia. This may last for more than a year.
While polyvalent intravenous immunoglobulin supplementation is routinely used in children, there is no consensus on its use in adult CAR-T recipients with hypogammaglobulinaemia. It can be considered in cases of infection with encapsulated bacteria in the presence of hypogammaglobulinaemia < 4 g/L.
Risk of infection
Patients receiving CAR-T cells are particularly immunocompromised and at risk of developing infections. In real-life studies, infectious complications affect 23%–42% of patients [25,26]. The secondary immune deficiency is multifactorial in origin: haemopathy itself, toxicity of the various previous lines of chemotherapy, previous auto or allograft, hypogammaglobulinaemia, neutropenia which is sometimes prolonged, toxicity of treatments, toxicity of lymphodepletion, inflammatory state linked to CRS, and corticosteroid therapy (used in particular in the treatment of ICANS, but also CRS in case of non-response to tocilizumab). The majority of infections occur within 30 days of CAR-T injection and are largely mild to moderately severe. Bacterial infections occur first, and are often early, followed by later viral infections. These viral infections are mostly caused by upper respiratory viruses, but cytomegalovirus (CMV) may also be present. Invasive fungal infections are rare and are mainly seen in patients with ALL, especially those who have received an allograft [25,26]. The occurrence of severe CRS is correlated with the risk of developing an infection [25,26]. If fever occurs soon after CAR-T injection, it is difficult to distinguish between CRS and an infectious complication. Most often, the situation is one of febrile aplasia requiring infectious samples (blood cultures from central and peripheral venous lines, cytobacteriological examination of the urine [ECBU] ± respiratory samples) and the immediate initiation of broad-spectrum empirical antibiotic therapy.
In terms of prophylaxis, the most recent recommendations recommend antiviral prevention with valaciclovir or aciclovir, antipneumocystis with cotrimoxazole (or pentamidine, or atovaquone in case of intolerance to cotrimoxazole). These prophylactics should be started at the time of lymphodepletion and stopped at one year after reinjection of CAR-T cells and/or until a CD4+ lymphocyte count of >200/mm3 is achieved. Antibiotic prophylaxis with fluoroquinolones (levofloxacin or ciprofloxacin) should only be discussed in cases of prolonged neutropenia [12].
In addition, it should be noted that any infection contraindicates the initiation of lymphodepletion, due to the risk of severe sepsis, as well as the reinjection of CAR-Ts, due to the risk of severe CRS and ICANS. It is therefore recommended to ensure that there is no active infection before starting the procedure [12]. Regarding immune reconstitution, in addition to neutropenia, which may be delayed, and B hypoplasia, which may last for more than a year, CD8+ T cell reconstitution occurs fairly early, in contrast to CD4+ T cells which may remain below 200/mm3 for up to two years.
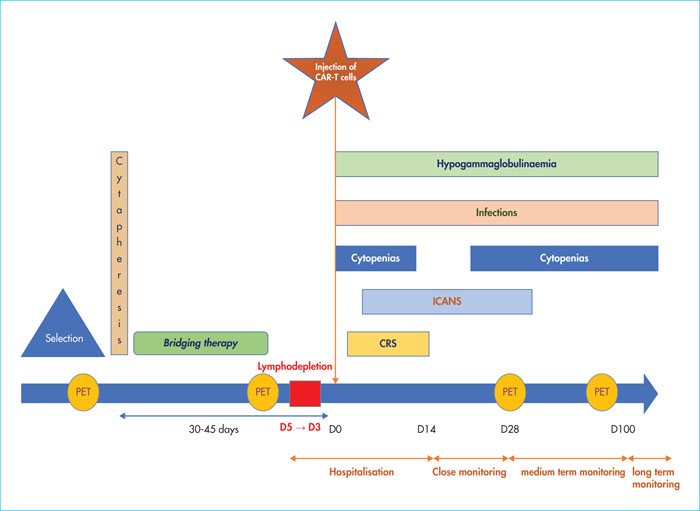
Figure 7 summarises the care pathway for CAR-T patients and shows the kinetics of the main complications.
Conclusion
The care pathway for patients, as part of the CAR-T administration programme, is formalised by a rigorous procedure involving a large number of actors. Patients must meet a number of eligibility criteria. The two main reasons for ineligibility are disease that progresses excessively and the existence of comorbidities. The management of the two main complications, CRS and ICANS, should follow treatment algorithms recommended by expert groups. Harmonisation of these practices may facilitate the management of these adverse effects on a daily basis.
Conflict of interest
CT has consulted and participated on the boards for Amgen, Celgene, Jazz Pharmaceutical, Kyte, Novartis, Servier and Roche, and has received funding from Roche, de Hospira and de Celgene.