Epileptic Disorders
MENUWomen's issues Volume 22, numéro 4, August 2020
This manuscript is based on the “Ask the Expert” session on “Women's issues” during the International Epilepsy Congress, Bangkok, Thailand, 2019. The purpose of the session and this manuscript were to address ILAE Competencies and Learning Objectives related to issues in the management of women with epilepsy (Blümcke et al., 2019). These issues include:
- –counselling women of childbearing age about the implications and management of epilepsy, providing guidance regarding pregnancy (including teratogenicity of the various antiepileptic drugs) and guidance regarding post-partum and child care;
- –demonstrating up-to-date knowledge about the range of pharmacological treatments for epilepsy, the spectrum of action for antiepileptic drugs, knowledge regarding adverse effects of antiepileptic drugs, and knowledge about treatment of psychiatry and cognitive comorbidities in children and adults;
- –and demonstrating the ability to diagnose and manage cognitive and psychiatric comorbidities, recognize psychiatric comorbidities (such as bipolar disorder, depression and mania), and appropriately manage or advise regarding psychiatric comorbidities.
Case 1. Decision on whether to continue treatment with valproate in a woman with juvenile myoclonic epilepsy who is considering pregnancy
Ravish Keni
A 27-year-old woman presented to our epilepsy clinic with seizures starting at 14 years of age without a history of prior problems. Her seizure semiology included myoclonic jerks on awakening and generalized tonic-clonic seizures (GTCS) precipitated by sleep deprivation. Her baseline EEG showed frontally dominant generalized fast polyspikes, with activation during hyperventilation and photic stimulation. Her electroclinical phenotype was consistent with a diagnosis of juvenile myoclonic epilepsy.
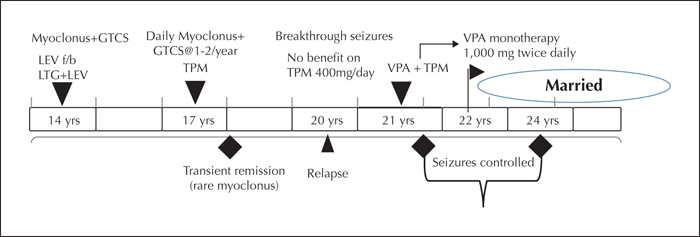
She was initially treated with lamotrigine at 200 mg/day and levetiracetam at 1,500 mg/day. She was well-compliant to medications and adhered to lifestyle measures that were advised. Facilities for therapeutic drug monitoring were unavailable in this rural set-up. She continued to have frequent myoclonus in the morning and GTCSs once or twice per year. A further increase in lamotrigine was not considered in view of disabling myoclonic jerks. On increasing the dose of levetiracetam, her family members reported significant behavioural issues, hence she was maintained on a dose of 1,500 mg per day with which these symptoms were fairly stable. She was subsequently switched to topiramate monotherapy (200 mg/day) and was seizure-free except for rare periods of myoclonus for two years. Seizures, however, returned and continued despite maximally tolerated doses of topiramate (400 mg/day). Valproate was then added after discussing with the patient about benefits versus potential adverse effects. Her seizures became well controlled on starting valproate. She was subsequently able to achieve transition to valproate monotherapy at 1,000 mg twice daily and has been seizure-free for one year. Her treatment details and clinical response are summarized as a timeline in figure 1.
She is recently married and is interested in becoming pregnant and would like to breastfeed her infant. She is concerned about the risks of valproate exposure to her developing infant.
Questions to the experts
- –What steps should be taken in the above case?
- –Is there any safe dose of valproate in pregnancy?
- –If a woman with epilepsy on valproate has conceived should you consider switching to alternative antiseizure medication?
Expert comments
The benefits of valproate include seizure control with reduced risks to the mother and fœtus from seizures. Risks of valproate include adverse teratogenic effects including congenital malformations and impaired neurodevelopmental outcomes (i.e., cognitive and behavioural impairments) (Meador et al., 2013; Tomson, et al., 2018). For example, malformation rates for valproate were about 10% vs. 2.8% for levetiracetam and 2.9% for lamotrigine in the EURAP study (Tomson et al., 2018). These findings have been confirmed based on a meta-analysis across multiple studies (Veroniki et al., 2017). Intermediate risks have been found for phenobarbital and topiramate. Teratogens work in a dose-dependent manner. Since ASMs (antiseizure medications) are among the most prescribed teratogens in women of childbearing age, it is not surprising that a dose-dependent effect on malformations has been seen for multiple ASMs (Tomson et al., 2015). Thus, lower ASM dosages reduce the risk of malformations. In general, malformation rates are higher for polytherapy (Thomas et al., 2017), but these may differ across specific polytherapies. Some studies have suggested that effects of certain ASMs (i.e., topiramate and valproate) predominate as adverse effects seen with polytherapies (Vajda et al., 2018). Other studies have suggested that ASM interactions are more complex (Keni et al., 2018). In addition to anatomical malformations, risks due to foetal exposure to ASMs include behavioural teratogenesis, for example, a 7-10 point reduction in IQ was reported for valproate compared to other standard ASMs (i.e., carbamazepine, lamotrigine, phenytoin) (Meador et al., 2013). These effects of valproate on cognition are also dose-dependent, but a dose as low as 800 mg/day has been shown to reduce verbal IQ and increase the need for special education (Baker et al., 2015). Levetiracetam has also shown better cognitive outcomes compared to valproate (Bromley et al., 2016). In addition, foetal valproate exposure has been associated with an increased risk of autism and autistic spectrum disorder (Christensen et al., 2013). The underlying mechanisms of the effect of foetal ASM exposure on neurodevelopment may be similar to those of foetal alcohol as, like alcohol, multiple ASMs can cause neuronal apoptosis in the immature brain (e.g., valproate, phenobarbital, and benzodiazepines) (Turski and Ikonomidou, 2012). Beyond neuronal apoptosis, the remaining neurons exhibit reduced synaptogenesis, which is likely the main factor adversely affecting neurodevelopment. Several ASMs have been shown not to cause neuronal apoptosis in the immature brain (e.g., carbamazepine, lamotrigine, levetiracetam, and topiramate), but only levetiracetam has been shown not to enhance apoptosis when combined with an ASM that causes apoptosis (Turski and Ikonomidou, 2012). Although many ASMs have not been tested in this model, polytherapy may pose an increased neurodevelopmental risk, although the risks associated with many specific ASM polytherapies are unknown.
Preconceptional counselling should emphasize the importance of periconceptional folate supplementation, which has been shown to improve cognitive outcomes and reduce risk of autism (Meador et al., 2013, 2020; Bjørk et al., 2018; Husebye et al., 2018). Since women are typically pregnant for weeks before they are aware of their pregnancy, they should take daily folate supplementation. The exact dose is not clear but should be at least 0.4-1.0 mg/day. Concern has been raised that high-dose folate (e.g., >4 mg/day) may result in increased risk of autism and other developmental disorders (Wiens and DeSoto, 2017). Thus, the optimal dose is unknown and it is unclear whether this dose differs between the healthy population and women taking ASMs.
In the above case with juvenile myoclonic epilepsy, there is no simple clear treatment choice. Thus, the decision has to be made in conjunction with the patient after delineating the relative risks and unknown factors for treatment options. In this case, the patient failed lamotrigine and topiramate, and 2,000 mg/day of valproate appeared to control her seizures; this is a dose with high teratogenetic risks. Options include addition and transition to levetiracetam, or the addition of lamotrigine in an attempt to reduce the daily dose of valproate, although even fairly low dosages of valproate still pose some risk. Other ASMs such as zonisamide and perampanel could be considered, but knowledge of teratogentic risks are limited for other ASMs that are effective against juvenile myoclonic epilepsy. Thus, such decisions are made with limited evidence base against known risks associated with valproate. The best time by far to consider ASM changes is prior to pregnancy. By the time a woman knows that she is pregnant, the risk of congenital malformations has already largely occurred. Changing ASM during pregnancy also entails the risk of precipitating seizures and putting both the mother and child at risk.
Case 2. Factors contributing to severe seizure worsening during the peri-partum and postpartum period in a patient with juvenile myoclonic epilepsy
Barbara Mostacci, Laura Licchetta
A 31-year-old woman had a family history of epilepsy (i.e., one sibling with juvenile myoclonic epilepsy, and one nephew and one niece with epilepsy onset during infancy). Her seizures started at 14 years, with jerks on awakening and sporadic GTCSs on sleep deprivation. Myoclonus disappeared with valproate at 500 mg/day, while GTCSs persisted. At 22 years, she was shifted to phenobarbital at 25 mg/day and myoclonus reappeared, while sporadic GTCSs persisted only after sleep deprivation. EEG and MRI were unremarkable. At 24 years, she was referred to us and was shifted to levetiracetam, titrated to 750 mg twice a day; blood level was 11.9 mcg/ml. She was subsequently seizure-free with the exception of a GTCS following a 48-hour levetiracetam withdrawal period.
Sixteen months later, she became pregnant. The team were largely in favour of maintaining the levetiracetam dose at >65% of the pre-pregnancy level, but the patient did not want to add more medication. Therefore, levels were maintained within the low range. Levetiracetam levels were 3.5 mcg/mL at the 14th week on 1,500 mg/day, 4.6 mcg/mL at the 21st week on 2,000 mg/day, 5.7 mcg/mL at the 27th week on 2,250 mg/day, and 4.9 mcg/mL at the 32nd week on 2,750 mg/day. Her dose was increased to 1,000 mg, three times a day, in the 31st week. Levetiracetam level in the 38th week was 23.3 mcg/mL on 3,000 mg/day, but this was drawn at peak unlike prior levels near the nadir. During the 38th and 39th weeks, she had two GTCSs during sleep. Levetiracetam dosage was increased to 3,750 mg/day, and a Caesarean section was performed. During the first three months after delivery, GTCS occurred several times per month, often triggered by sleep deprivation, despite adding clobazam at 20 mg/day and valproate at 1,250/day. On two occasions, GTCS occurred in clusters (12 seizures in 24 hours the first time and nine seizures in the second) requiring intravenous benzodiazepines. The EEGs performed during this period showed background slowing and abundant generalized epileptic discharges. After the second cluster of seizures, phenobarbital at 75 mg/day was added. During the following year, she had three GTCSs occurring after sleep deprivation during menses. Clobazam, valproate and phenobarbital were slowly withdrawn and lamotrigine was added. During lamotrigine titration, she became pregnant. Pregnancy was uneventful, and she has had only one GTCS after lamotrigine introduction, over the following three years. EEGs normalized. She is currently on levetiracetam at 2,000mg/day (blood level: 16.2 mcg/mL) and lamotrigine at 500mg/day (9.9 mcg/mL).
Questions to the experts
- –This was a remarkable worsening limited to the peri-partum and postpartum period, apparently not entirely attributable to the drop in levetiracetam blood levels, nor self-perceived sleep deprivation. What are the potential causes?
- –Can patients with juvenile myoclonic epilepsy be more susceptible to this kind of worsening? Is it worth adopting a more cautious approach for this condition?
- –In which situations would you advise a Caesarean section?
- –Is there any particular advice for breastfeeding in patients who have been proven to be very susceptible to sleep deprivation?
- –Should we still advise breastfeeding when the baby is exposed to a new drug or combination of drugs which were not taken during pregnancy?
Expert comments
The first question implies that the peri-partum and postpartum seizure exacerbation was not due to a drop in levetiracetam level or self-perceived sleep deprivation. Changes in levetiracetam levels during pregnancy are variable, but on average, one can expect a 200% increase in clearance (Voinescu et al., 2018). The therapeutic range of levetiracetam is not completely established but is approximately 10-45 mcg/mL, although some patients are treated at even higher levels. In this case, the two reported levels in the non-pregnant state were 11.9 mcg/mL on 1,500 mg/day before the first pregnancy and 16.2 mcg/mL on 2,000 mg/day after the second pregnancy. These are in the low therapeutic range. The levels during pregnancy were low except the last level drawn at peak, which was within the lower mid therapeutic range, and the trough levels could have been subtherapeutic. Subsequently, the dose was increased to 3,750 mg/day, although it is not clear whether this is an adequate level for this patient. This might explain the seizure exacerbation in the peri-partum and early postpartum period, however, within 1-2 weeks postpartum, most of the metabolic change associated with pregnancy reversed, and the levetiracetam level should have increased. The later combination of levetiracetam and lamotrigine appeared to control her well in her subsequent pregnancy.
During the three months postpartum, it was noted that seizures were often triggered by sleep deprivation. Sleep deprivation is the most common seizure trigger in the postpartum period. There needs to be a plan to assure adequate sleep for the mother. This plan may require pumping of breastmilk or supplementation of bottled milk at night given by a family member to allow the mother to sleep. There should also be safety precautions for newborns such as using a sling to carry the baby, and other measures to avoid the mother carrying the baby in her arms or bathing the baby alone.
There is no evidence to indicate that juvenile myoclonic epilepsy patients are more susceptible to seizures in the postpartum period than those with focal epilepsy or other generalized epilepsies. Both generalized and focal epilepsies are susceptible to sleep deprivation. Other potential contributors to seizure exacerbation could be stress or peripartum hormonal fluctuations, but the data supporting the notion that these factors contribute to seizures in the peri-partum and postpartum period are inadequate.
Caesarean section could be considered for patients with multiple seizures near term or seizures during labour when the mother does not recover quickly. Otherwise, Caesarean sections should be for standard obstetric indications.
There are multiple benefits to both the mother and child from breastfeeding (Ip et al., 2009). In the past, women on antiseizure medications were advised not to breastfeed out of concern that drug exposure via breastmilk could adversely affect the child's neurodevelopment. Two studies examining neuropsychological outcomes in children aged three years, after breastfeeding by mothers taking antiseizure medication, found no adverse effects (Meador et al., 2010; Veiby et al., 2013); in one of these studies, the cohort was followed to age six years and improved cognitive outcomes were even reported in those children who were breastfed (Meador et al., 2014). Given the known benefits of breastfeeding, it seems reasonable to recommend breastfeeding when the baby's mother is taking an ASM since the only studies available provide no evidence of harm due to ASMs during breastfeeding.
Cases 3. Management of psychiatric comorbidity in a young woman with epilepsy and bipolar disorder
Gordana Kiteva-Trenchevska, Liljana Ignjatova
A young woman with epilepsy and comorbid bipolar disorder - type one (BPD-I)- is presented. Although there was no family history of epilepsy, her older sister had febrile seizures at the age of 10 months. Her family history was positive for BPD (her older sister and grandmother). She was a 22-year-old student who started to manifest her seizures at the age of 18 years. The diagnosis was not clear initially as her seizures were unwitnessed. Several investigations were performed showing normal findings (standard laboratory bloodwork, ECG, EEG, and brain MRI). As the patient was found to be depressive, it was assumed, incorrectly, that the seizures were non-epileptic psychogenic spells. Antidepressant treatment, i.e., selective serotonin reuptake inhibitor (SSRI), was prescribed. However, the seizures continued and were recognized as GTCS (although it was unknown whether seizure onset was focal or generalized). There was no aura or other focal manifestations at seizure onset. Repeat EEG and MRI were normal. The diagnosis of epilepsy was made, and the antidepressant was gradually withdrawn. Lamotrigine was introduced and slowly titrated up to a daily dose of 300 mg, but GTCS continued. Additional laboratory findings revealed latent hypothyroidism with increased levels of anti-thyroid peroxidase antibodies, therefore levothyroxine, 25 mg daily, was added to her treatment. Despite normalization of laboratory findings, seizures continued, and the patient was hospitalized. Two GTCS occurred during the first days of hospitalization, and she developed symptoms of mania (i.e., talkative with flight of ideas, ideas of grandiosity, full of energy, singing, drawing pictures in her notebook, writing novels in English, and loss of sleep). There was no substance abuse. Her psychological state showed mania, and she was diagnosed with a manic episode of BPD-I and epilepsy with GTCS of unknown onset. Laboratory findings, including thyroid hormones and thyroid-stimulating hormone, were normal. A repeat routine EEG and prolonged EEG after sleep deprivation were normal. Repeat brain MRI was normal.
The next challenge was which drugs should be used for seizure control and for acute treatment of the manic phase of BPD. Valproate was gradually introduced at 500 mg twice daily, lamotrigine gradually withdrawn and reduced to 50 mg twice daily, and antipsychotic quetiapine, 50 mg twice daily, was added for sleep loss. During follow-up, her mental status improved, with no seizures.
Questions to the experts
- –What is the rationale for an individualized approach for acute treatment of epilepsy and BPD-I?
- –How is an informed decision process achieved in the setting of mania? Should the doctor or patient know what the best treatment option is?
- –What would be your decision for future long-term maintenance treatment of this patient for seizure and BPD-I control in the context of possible future pregnancy?
Expert comments
There are multiple options for the treatment of epilepsy with BPD comorbidity in women of childbearing potential. Although studies on the relationship between epilepsy and BPD have been published (Mazza et al., 2007; Sucksdorff et al., 2015), controlled trials with women with epilepsy and BPD are lacking. Thus, the evidence base for treatment is inadequate to fully direct therapy. Some ASMs are used for seizure control and at the same time as mood stabilizers for mood disorders such as BPD (valproate, lamotrigine, carbamazepine, and oxcarbazepine). For some ASMs (e.g., lamotrigine and levetiracetam), the risk of major congenital malformations (MCMs) and the risk of poor neurodevelopmental outcome in children of women of childbearing potential are low (Tomson et al., 2018), however, the risk associated with foetal exposure to most ASMs is uncertain regarding both anatomical and behavioural deficits (Meador and Loring, 2016). As discussed before, intrauterine valproate exposure is associated with a higher and dose-dependent risk of foetal teratogenicity (Harden et al., 2009; Bromley et al., 2014; Tomson et al., 2015, 2018), and it is recommended that valproate be avoided in female patients of childbearing potential (European Medicines Agency, 2018), however, valproate may be potentially life-saving for some mothers with epilepsy (Thomas, 2018). First-line treatments for BPD-I are lithium, ASMs, and antipsychotics. However, some ASMs are not recommended for treatment of acute mania, such as lamotrigine, topiramate, zonisamide, and eslicarbazepine (Yatham et al., 2018). The risk of hypothyroidism is increased in patients with BPD (Lambert et al., 2016) and this should be taken into consideration when selecting mood stabilizers for patients with subclinical hypothyroidism.
In the above case, lamotrigine, 300 mg daily, did not result in seizure control, and the patient also suffered from mania. The decision was made to withdraw lamotrigine as it can provoke mania, although it is a good stabilizer for depression. Valproate was introduced, lamotrigine reduced, and quetiapine added, resulting in control of seizures and BPD. Going forward, a discussion of risks related to a potential future pregnancy is required to provide adequate informed consent to this young woman. She needs to understand the teratogenic potential of malformations and neurodevelopmental adverse effects associated with foetal valproate exposure (Bromley et al., 2014; Veroniki et al., 2017) and that psychiatric disorders are associated with epilepsy, related to increased morbidity and even mortality (Fazel et al., 2013). Furthermore, she should be aware of the risk of bipolar disorder or seizures relapsing upon valproate withdrawal or dose reduction before or during pregnancy (Tomson et al., 2016; Yatham et al., 2018).
In this case of a woman with GTCS with focal or generalized epilepsy, a broad spectrum of ASMs is indicated. Levetiracetam is a broad-spectrum antiseizure medication with minimal risk of teratogenicity, but the risk of provoking psychiatric side effects with levetiracetam in this patient with BPD-I is a potential concern. However, levetiracetam could be an option for seizure control in combination with a drug for BPD-I treatment. Other potential broad-spectrum ASMs include topiramate, associated with intermediate teratogenic risk, and perampanel, for which there is no information in humans on teratogenic risk, however, both can produce adverse behavioural effects. There are other ASMs with potential broad-spectrum activity, but these are not approved for generalized epilepsy and there is little data available on teratogenic risk (e.g., lacosamide and zonisamide). Carbamazepine or oxcarbazepine are ASMs that are also mood stabilizers, which also have favourable teratogenic profiles. However, they are effective for focal but not generalized epilepsy. Therefore, these ASMs are not a first option in this patient, but could be effective if the GTCSs are focal to bilateral tonic-clonic seizures. There is no clear-cut choice for future long-term maintenance treatment in this patient for seizure and BPD-I control. Therapeutic decisions should be made jointly between the doctor and a well-informed patient in order to make the best decision for the individual. In the setting of mania, the delusional state may interfere with full participation by the patient. However, once in remission, decisions on long-term maintenance treatment should be addressed jointly.
Conclusions
Treatment of epilepsy in women of childbearing potential requires special consideration. A suggested flowchart is offered (figure 2). It is important, prior conception, to select appropriate ASMs for seizure control as seizures could harm the mother and the foetus. An individualized and holistic approach for each patient should be made with consideration of comorbidities in order to reduce maternal mortality and morbidity during pregnancy, as well as maximize the outcome for the mother's offspring. If possible, ASMs should be selected with minimal adverse effects for the mother and foetus. The challenge is even greater if the woman has failed multiple medications or if the woman's epilepsy is associated with comorbidity with the need for chronic treatment of both conditions. In women with medically resistant epilepsy, evaluation and consideration of possible epilepsy surgery should be pursued.
Key points
- •Women with epilepsy should be informed of the teratogenic risks associated with antiseizure medications prior to pregnancy, preferably at the time of the first drug prescription.
- •Preconceptional recommendation should include the routine use of folate to reduce the risk of poor developmental outcomes in the offspring of women with epilepsy.
- •Valproate poses a particular teratogenic risk of both anatomical (i.e., congenital malformations) and behavioural (e.g., impaired cognition and increased risk of autism) deficits. It should be avoided when possible in women of childbearing potential.
- •Concerns for teratogenic risks need to be balanced along with the risks associated with seizures in the mother and child.
Supplementary data
Summary didactic slides are available on the www.epilepticdisorders.com website.
Acknowledgements and disclosures
The authors wish to thank the ILAE for their support of the prior presentation and development of the present manuscript.
Dr. Mostacci has received speaker or consultant honoraria from EISAI, SANOFI, UCB Pharma, Univadis and travel support from LivaNova and EISAI. Dr. Thomas has received research grants from the DST (SAN No. 102/IFD/SAN/156/2017-2018) and ICMR (5/4-5/152/Neuro/2015-NCD-1) for the Kerala Registry of Epilepsy and Pregnancy and honoraria for lectures from British Medical Journal India. Dr. Meador has received research support from the National Institutes of Health and Sunovion Pharmaceuticals, and travel support from UCB Pharma. The Epilepsy Study Consortium pays Dr. Meador's university for his research consultant time related to Eisai, GW Pharmaceuticals, NeuroPace, Novartis, Supernus, Upsher-Smith Laboratories, UCB Pharma, and Vivus Pharmaceuticals. Dr. Kani, Dr Kiteva-Trenchevska, Dr. Licchetta and Dr. Ignjatova have no conflicts of interest to disclose.
* This work was previously presented at the “Ask the Expert” session on “Women's issues” during the International Epilepsy Congress, Bangkok, Thailand, June 2019.
![]() Cette œuvre est mise à disposition selon les termes de la
Licence Creative Commons Attribution - Pas d'Utilisation Commerciale - Pas de Modification 4.0 International
Cette œuvre est mise à disposition selon les termes de la
Licence Creative Commons Attribution - Pas d'Utilisation Commerciale - Pas de Modification 4.0 International