Epileptic Disorders
MENUWhen the brain hurts the heart: status epilepticus inducing tako-tsubo cardiomyopathy Volume 21, numéro 3, June 2019
Tako-tsubo cardiomyopathy (TTC) is a transient myocardial systolic dysfunction, mainly affecting the left ventricle that can mimic an acute coronary syndrome. This condition was firstly described in 1990 in Japan, and its name is related to the echocardiographic and ventriculographic shape of the heart that resembles a Japanese pot used to catch octopus (tako: octopus; tsubo: trap) (Dote et al., 1991). It can be precipitated either by psychologically/physically stressful events (e.g. a surprise, an unexpected death, etc.) or by a number of medical conditions (subarachnoid bleeding, traumatic brain injury, sepsis, surgical procedures, asthma exacerbation, hypoglycaemia, exercise, etc.) (Bybee et al., 2004; Finsterer and Bersano, 2015), however, a clear trigger is not always identified. Neurological diseases are strongly associated with TTC, and among neurological conditions, the strongest association is found with subarachnoid haemorrhage followed by status epilepticus (SE) (Stöllberger et al., 2011; Sutter et al., 2018; Morris et al., 2019).
The pathogenesis is not completely determined but one of the most frequently advocated mechanisms is a direct or an indirect effect of excessive catecholamine release on myocardial function, creating a “stunned myocardium” (Stöllberger et al., 2011). Post-menopausal women seem to be at high risk, probably due to the loss of the protective effect of oestrogens on the function of the myocardium (Yoshikawa, 2015).
International consensus on diagnostic criteria for TTC is still lacking. Since its first description, many different diagnostic criteria have been proposed and subsequently updated (Bybee et al., 2004; Kawai et al., 2007; Prasad et al., 2008; Wittstein, 2012; Redfors et al., 2013, 2014; Parodi et al., 2014).
Clinically, TTC can be characterized by an acute onset of chest pain and dyspnoea, but is frequently completely asymptomatic and easily unrecognized. Nevertheless, in some cases, it can evolve into pulmonary oedema, arrhythmias, intraventricular thrombus with risk of cardio-embolic stroke, and cardiogenic shock requiring intensive care unit (ICU) admission and intensive care management (amine support and diuretic therapy), thus becoming a life-threatening condition (risk of lethal arrhythmia or septal perforation) (Mrejen-Shakin et al., 2011; Wakabayashi et al., 2011).
Creatine kinase (CK), the isoenzyme CK-MB, troponin, myoglobin, and brain natriuretic peptide (BNP) can be transiently and mildly/moderately elevated. Even though the increment of myocardial necrosis enzymes is mild to modest according to the proposed criteria, it has been recently suggested that their level is related to the level of affected myocardium.
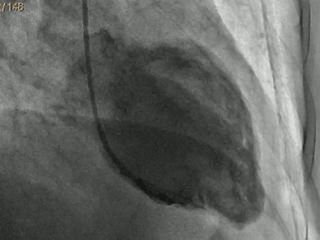
Thus, the level of myocardial necrosis enzymes may increase if a large part of the left ventricle is affected (Parodi et al., 2014). Initial electrocardiography (ECG) frequently shows alterations such as diffuse ST elevation, T-wave inversion, prolonged QT interval, or abnormal Q waves. Echocardiograms show wall motion abnormalities (WMA) of the left ventricle consisting of hypokinesia/akinesia, mostly confined to the apex, mid-left ventricular segments (classic type), and mid-left ventricular segments only (mid-ventricular type), and are sometimes associated with compensatory hyperkinesia of the basal segments; basal and mid-left ventricular segments (inverted type) or all segments (global type). Moreover, in some rare cases, the right ventricle may also be involved (Yoshikawa, 2015). In all cases, the hypokinesia/akinesia goes beyond a single epicardial vascular distribution and is accompanied by a reduction in ejection fraction (EF). The coronarography is generally unremarkable or shows non-obstructive epicardial coronary artery disease. After the acute phase, the evolution is mostly favourable with normalization of myocardial function within days or months (Redfors et al., 2014).
We hereby present three new cases of TTC induced by SE and review published cases in order to evaluate the main features of this condition in patients with SE and the potential complications.
Material and methods
Personal cases
The data for cases fulfilling the diagnostic criteria for TTC (see the adopted criteria described in box 1) among all consecutive episodes of SE observed in young adults and adults (≥14 years old), from September 1st 2013 to the end of April 2018, were retrospectively collected and reviewed. Patients were observed at the Ospedale Civile Sant’Agostino Estense of Modena (regional centre for neurological diseases for Modena city and district, Italy).
SE episodes were prospectively collected during the above-mentioned period using a specific case report form. The form was initially completed by the first doctor who took care of the patient (in the majority of cases, a neurologist or a neuro-intensivist or a physician in the emergency room) or by the staff of the neurophysiology unit who performed the first EEG examination of a suspected SE case (< 24 hours in every case). A neurology ward serves the hospital 24 hours/day, seven days/week and the same neurophysiology staff records all the EEGs. A neurologist, trained in the field of epilepsy, then reviewed all forms, EEGs, and completed any missing information by consulting the hospital informatics database.
Literature review
As this condition has been given many different names, we used a variety of search strategies. We searched MEDLINE (accessed through PubMed from inception to August 31st, 2018) using the following terms: “status epilepticus” OR “epilepsy” AND “takotsubo cardiomyopathy”, “takotsubo syndrome”, “tako-tsubo cardiomyopathy”, “tako-tsubo syndrome”, “stress cardiomyopathy”, “myocardial stunning”, “ampulla syndrome”, “left ventricular apical ballooning”, “broken heart syndrome”.
All titles and abstracts were evaluated, and any relevant article was considered without any restriction of language. In all the included articles, the references were hand-searched to identify any further study which was not previously considered. In all included articles, we collected information about the demographic and past medical history of the patients, the characteristics of the SE (type, aetiology, EEG characteristics, and therapeutic response), the clinical and instrumental characteristics of the acute phase (presenting symptoms, biochemical tests, ECG, echocardiogram, coronarography features), the type of acute management and the complications of the acute phase, and the clinical and instrumental evolution.
Using “takotsubo cardiomyopathy and status epilepticus” or “takotsubo syndrome and status epilepticus” or “tako-tsubo cardiomyopathy and status epilepticus” or “tako-tsubo syndrome and status epilepticus”, we obtained 21 results. One study was excluded because an abstract was not available, two were reviews, and one was a cross-sectional study not reporting new cases. Four more articles were excluded after having read the abstract because they were judged to be unrelated. Therefore, 13 papers were finally included. “Stress cardiomyopathy and status epilepticus” led to 22 results (13 already included, eight already excluded, and a review excluded). “Myocardial stunning and status epilepticus” led to three results (one already included, one excluded after reading the abstract because the authors attributed the myocardial dysfunction to pentobarbital infusion instead of SE, and one other paper included). “Ampulla syndrome and status epilepticus” did not lead to any results. “Left ventricular apical ballooning and status epilepticus” led to five results (four already included and one already excluded). Finally, “Broken heart syndrome and status epilepticus” led to 21 results (13 already included and eight already excluded). With the same search strategy terms, we also checked the reviews and added five more papers reporting cases of SE-induced TTC not already included (for two of them, only an abstract was available). Moreover, we searched with each of the following terms “takotsubo cardiomyopathy”, “takotsubo syndrome”, “stress cardiomyopathy”, “myocardial stunning”, “ampulla syndrome”, “left ventricular apical ballooning”, “broken heart syndrome” together with “epilepsy” to check whether there were any other cases of TTC induced by SE not included in the analysis, however, this was not the case.
Results
Personal cases
In our department, we recorded 392 episodes of SE from the beginning of September 2013 to the end of April 2018. In our cohort, among the 392 SE episodes, 85 (22%) were classified as generalized convulsive SE (GCSE) forms with or without subsequent evolution into a non-convulsive form. Overall, there were 223 (57%) cases of SE with major motor phenomena (either generalized or focal), while 169 (43%) were NCSE.
Overall, we observed three cases of TTC resulting in an incidence of 0.77%.
Case 1
A 74-year-old woman was admitted to our neurology department because of a first tonic-clonic seizure followed by prolonged aphasia. The past medical history was remarkable only for mild arterial hypertension without any cardiological involvement.
The EEG showed a left frontal SE promptly responsive to diazepam, followed by phenytoin and valproic acid treatment. Brain MRI showed a left fronto-insular neoplasm. Two days after admission, the patient experienced chest pain and dyspnoea. The ECG showed diffused T-wave inversion and a prolonged QTc interval (537 msec) (figure 1). The echocardiogram showed diffused akinesia (apical, medial segment, septum, anterior and inferior walls) with a concomitant decreased EF (38%). Myocardial necrosis enzymes were slightly elevated (CK-MB: 6.5 ng/ml, normal value (nv): 0.6-6.3 ng/ml; myoglobin: 334 ng/ml, nv: 15-66 ng/ml; troponin: 0.89 ng/ml, nv:
Eight days later, an echocardiogram showed complete regression of the akinesia with normal wall motion and increased EF to 50%. Myocardial necrosis enzymes became negative in the subsequent 15 days while the ECGs were still abnormal 10 days later. Inverted T waves disappeared only two months later. At hospital discharge, the patient was on valproic acid.
According to the latest ILAE classification of SE (Trinka et al., 2015), a diagnosis of focal non-convulsive SE (NCSE) without coma was made (aphasic SE; progressive symptomatic SE due to a low-grade glioma).
Case 2
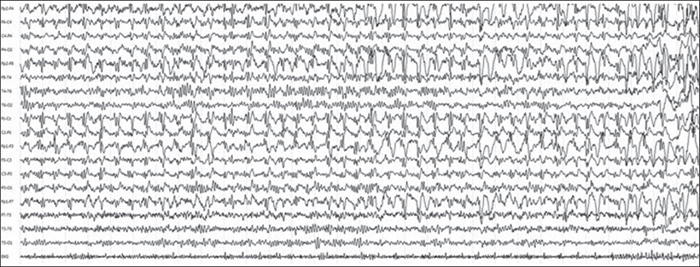
A 63-year-old woman presented to the emergency department because of sudden onset of two tonic-clonic seizures, followed by prolonged psycho-motor agitation and mental confusion. The first EEG showed repetitive, continuous spike-wave discharges at the fronto-central right leads with a rapid spatio-temporal evolution to a bi-frontal distribution, therefore a diagnosis of NCSE was made and valproic acid was administered (figure 2). In the previous days, she had abruptly stopped treatment with olanzapine for a known bipolar disorder. The past cardiological history and brain MRI were unremarkable.
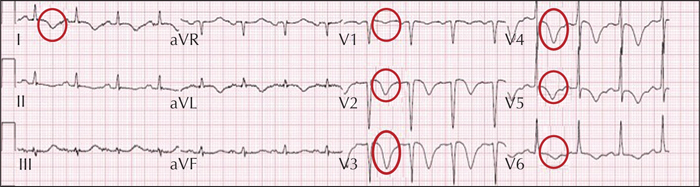
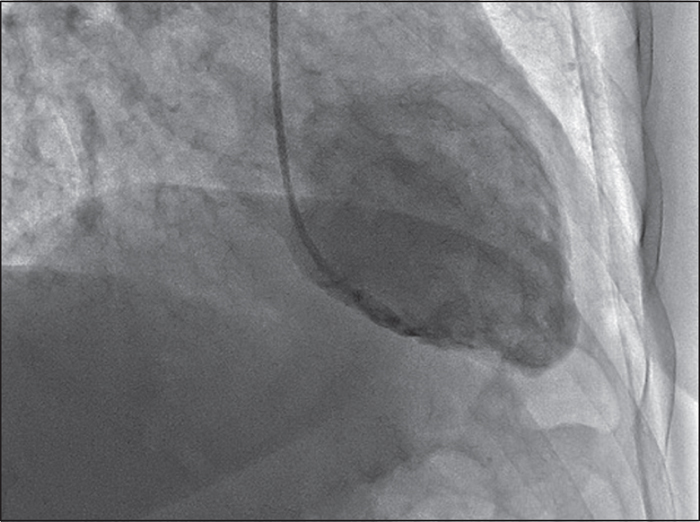
At the time of admission to the emergency room, she did not have dyspnoea or chest pain. The first ECG showed anterior T-wave inversion and the troponin levels were markedly altered (1,200 ng/ml; nv: figure 3and video). Antiplatelet therapy, β-blockers, and diuretics were started. At hospital discharge, the patient was still on valproic acid. Nineteen days later, troponin levels and ECG recovered. A diagnosis of mid-ventricular tako-tsubo was made.
According to the current ILAE classification of SE (Trinka et al., 2015), a diagnosis of focal NCSE without coma but with impaired consciousness of unknown origin was made.
Case 3
A 65-year-old woman was admitted to our neurology department because of a first episode of GCSE responsive to benzodiazepine treatment, due to right parieto-temporal cerebral metastasis. Her past medical history was remarkable for a diagnosis of lung adenocarcinoma and surgery for atrio-ventricular canal correction. Upon routine ECG, a lateral T-wave inversion was found. The patient was completely asymptomatic for chest pain or dyspnoea. The echocardiogram showed akinesia of the septum, inferior, and posterior walls, and a reduced EF (38%), while troponin was slightly elevated (1.86 ng/ml; nv:
According to the current ILAE classification of SE (Trinka et al., 2015), a diagnosis of focal onset evolving into bilateral convulsive SE was made (aetiology: progressive symptomatic due to cerebral metastasis).
Literature review
Based on the literature review, we included 19 papers (Sakuragi et al., 2007; Shimizu et al., 2008; Bosca et al., 2008; Legriel et al., 2008; Seow et al., 2008; Lemke et al., 2008; Fugate et al., 2009; Benyounes et al., 2011; Mrejen-Shakin et al., 2011; Rodriguez de Antonio et al., 2011; Traullé et al., 2011; Wakabayashi et al., 2011; Finsterer et al., 2013; Hocker et al., 2013; Belcour et al., 2015; Koo et al., 2015; Srivastava et al., 2016; Uemura et al., 2016; Miller et al., 2017) comprising a total of 42 cases. Together with our patients, 45 cases of TTC induced by SE were reviewed (see table 1 for a summary of the studies and supplementary tables 1-3 for details of each study/patient). The main findings observed with TTC are presented in table 1.
Overall, 28 (62%) patients were female. The median age was 60 years (ranging from 14 to 83 years). When reported, the SE was frequently of a convulsive type (36/40; 90%). Over a half of patients (13/22; 59%) had no previous history of epilepsy. The SE was of remote symptomatic aetiology in half of the cases (50%) and was mostly refractory to AED therapy (24/42; 57%). In our cases, TTC occurred during SE and the cardiac function was impaired for a variable period after cessation of the SE. This was similar to cases reported in the literature, even though the exact timing of TTC occurrence in relation to SE is rarely reported. The presenting symptom of TTC was most frequently arterial hypotension (30/43; 70%), and chest pain and dyspnoea (4/43; 9%) were also reported, whereas six patients were completely asymptomatic (14%). Moreover, severe presentation was reported in three cases with cardiogenic shock and ventricular fibrillation. Sixteen studies reported the need to start therapy with: amines (19 cases; 53%), diuretics (eight cases; 22%), antiplatelets (four cases; 11%), anticoagulation drugs (two cases; 6%), β-blockers (six cases; 17%), ACE inhibitors (four cases; 11%), and nitrates (two cases; 6%).
In the acute phase, ECG abnormalities in the form of ST elevation (31%), ST depression (5%), QTc prolongation (17%), and/or T-wave inversion (38%) were reported in the majority of patients. Overall, the echocardiography showed a diffuse decrease in EF (ranging from 15 to 49%). WMA and dilatation were classified as “classic type” in 58% of cases, “global type” in 30% of cases, and “mid-ventricular type” in 12%.
The values for myocardial necrosis enzymes and BNP were seldom reported but when reported were slightly to moderately elevated. Twenty-one patients (47%) underwent coronarography in order to rule out an acute coronary syndrome; in the vast majority of cases (90%), the findings were normal and in two cases (10%) the location of hypokinesia was discordant with the focal stenosis or the stenosis was not significant. The ventriculography confirmed the ecocardiographic findings.
The follow-up tests showed that all patients had either a complete recovery (91%) or a rapid improvement (9%) of cardiac function within weeks/months of the TTC diagnosis. Overall, severe complications either at clinical presentation or afterwards were reported in eight (19%) patients (pulmonary oedema, cardiogenic shock, ventricular fibrillation, and giant apical thrombus), but all of them had a complete recovery after ICU admission.
Discussion
Tako-tsubo cardiomyopathy is a rare consequence of SE. In our department, TTC occurred in about 1% of SE episodes. Even though TTC had a good outcome in the majority of the reported cases, rare potential life-threatening complications can occur.
We believe that this condition, especially in patients with SE, could be commonly overlooked and underdiagnosed due to the frequently reduced level of consciousness of these patients that prevents them from complaining about the presenting symptoms, such as chest pain or dyspnoea, as suggested in the case series of the French group (Belcour et al., 2015) and in the American cross-sectional study (Morris et al., 2019). Moreover, CK is frequently elevated from skeletal muscular lysis related to seizures, and only a focused search for CK-MB or troponin levels can reveal this condition.
Based on the cases in the literature as well as our cases, we can affirm that women over 60 are at highest risk of developing TTC. This is possibly related to endothelial dysfunction induced by reduced oestrogen levels, typical of the post-menopausal period, and to increased vasomotor reactivity in response to catecholamine-mediated stimuli (Bybee et al., 2004; Morris et al., 2019). The type of SE is not always reported in the literature. Nevertheless, when reported, SE associated with major motor manifestation is the most frequent type of SE related to TTC. Indeed, among the previously reported cases (42), 11 were classified as having GCSE. Overall, motor forms comprised 33 (79%) cases and four (9%) were NCSE, whereas for five (12%) episodes, the type of SE was not reported. We can assume that the level of release of catecholamines related to prolonged convulsions is higher than that in non-convulsive forms even though this requires further confirmation (Finsterer and Bersano, 2015). Based on this assumption, it is possible that in our first two cases, the initial convulsion caused the TTC.
Although in the great majority of patients, TCC has a benign course leading to a complete recovery, there are some reports of life-threatening complications such as cardiogenic shock, pulmonary oedema, arrhythmias, and left ventricle thrombus that could lead to stroke (Mrejen-Shakin et al., 2011; Wakabayashi et al., 2011). Moreover, catecholamine-related myocardial injury may be a relevant mechanism of sudden unexpected death in epilepsy (SUDEP) (Nashef et al., 2012; Al-Najafi and Rosman, 2015; Miller et al., 2017) even though this seems to be a rare event (Stöllberger et al., 2011; Finsterer and Bersano, 2015). Central modulation of the cardiovascular autonomic nervous system involves a complex network that includes the anterior insular cortex, the anterior cingulate gyrus, the amygdala, and the ventromedial prefrontal cortex. Some of these areas could be involved in the generation and propagation of seizures in a patient with SE and their involvement can promote catecholamine release. This could explain an increased risk of transient cardiomyopathy in TTC. However, ictal malignant cardiac arrhythmias are rare during the recorded cases of SUDEP. Nevertheless, it seems reasonable that ictal cardiac changes could play a significant role in patients who simultaneously experience respiratory distress and autonomic instability (Bermeo-Ovalle et al., 2015; Akashi et al., 2008; Ellis and Szabó, 2018).
Thus, clinicians dealing with seizures and SE in particular should keep TTC in mind in order to make a quick diagnosis and manage it, avoiding further complications. Moreover, it is important to avoid the use of antiepileptic drugs (in particular, ion channel blockers) that are known to interfere with cardiac function because of the potential increased risk of further cardiac complications (Bermeo-Ovalle et al., 2015).
Limitations of the study
We found that TTC was present in about 1% of the observed SE episodes. We believe that this percentage is underestimated. Indeed, the most important limitation of the present study is that we did not systematically investigate each patient with SE to rule out TTC. Thus, we identified only the symptomatic forms, assuming that probably more common asymptomatic forms of cardiac alterations could have been unrecognized.
Supplementary data.
Summary didactic slides and supplementary tables are available on the www.epilepticdisorders.com website.
Disclosures
None of the authors have any conflict of interest to declare.
* This work has previously been presented as a poster at the 13th ECE, Vienna.