Epileptic Disorders
MENUVideo‐EEG evidence of lateralized clinical features in primary generalized epilepsy with tonic‐clonic seizures Volume 5, numéro 3, September 2003
Auteur(s) : Leanne Casaubon, Bernd Pohlmann-Eden, Houman Khosravani, Peter L. Carlen, Richard Wennberg
Krembil Neuroscience Centre, Toronto Western Hospital, University of Toronto, Toronto, ON, Canada
Received May 26, 2003; Accepted July 3, 2003Presented in part at the American Epilepsy Society meeting, December 2002, Seattle, WA, USA
Primary generalized epilepsy (PGE; also idiopathic) is
characterized by seizures showing bilaterally synchronous,
generalized spike-and-wave activity recorded by
electro-encephalography (EEG) with clinical manifestations
including some, or all of, absences, myoclonic absences and/or
myoclonic jerks, and generalized tonic-clonic seizures. Whether the
cerebral cortex or subcortical structures, specifically the
thalamus, plays the dominant role in generating these seizures has
been the subject of long debate. Thalamic onset of generalized
seizures was originally hypothesized, supported by experimental
animal models of epilepsy and some human studies, and provided the
initial evidence that primary generalized seizures were the product
of bilateral reciprocal connections between specific thalamic
nuclei and a hyperexcitable cortex. There is now mounting evidence
to support a focal cortical generator as the initiator of cortical
hyperexcitability and generalized seizures [1, 2]. Reverberating
cortico-thalamo-cortical circuits, through thalamic structures that
have a propensity for rhythmic oscillations, allow for maintenance
of spike-and-wave activity and propagation of the seizure in a
generalized fashion. However, evidence for the exact nature and
localization of a focal cortical generator is lacking.
Very few patients with PGE undergo video-EEG monitoring, likely
given the usual clarity of clinical diagnosis and generally
excellent response to monotherapy with valproic acid, and the
clinical features of generalized tonic-clonic seizures in PGE are
typically based on historical information alone. Whether these
seizures truly have a generalized onset or a focal onset that
rapidly secondarily generalizes is therefore not clear. Some
reports in the literature have provided evidence to support a focal
clinical onset in some patients with PGE [3-5]. We report three
patients who underwent video-EEG monitoring whose ultimate
diagnosis was PGE based on clinical and EEG criteria; interestingly
all six recorded generalized tonic-clonic seizures commenced with
definite lateralizing features.
Methods and patients
From 300 patients referred to the Epilepsy Monitoring Unit
(EMU) at the Toronto Western Hospital, University of Toronto, for
continuous video-EEG investigation of refractory epilepsy, three
patients were found to have PGE with tonic-clonic convulsions.
Interictal and ictal EEG recordings and the corresponding video
sequences were reviewed.
Patient 1. J.L. presented at 19 years of age with a
five-year history of seizures, including generalized tonic-clonic
seizures and brief episodes of staring with occasional bilateral,
symmetric myoclonic jerks. He is the product of a normal pregnancy
and delivery, and early development was normal. From the history
there is no account of febrile or childhood seizures; there is a
strong family history of epilepsy. Previous investigations included
normal magnetic resonance imaging (MRI) of the brain and routine
EEG reported to show brief episodes of spike-and-wave activity with
a possible left frontal predominance. Formal neuropsychological
testing revealed a superior intellect and no lateralizing
abnormalities. He had been treated with carbamazepine monotherapy
for five years with modest seizure control. On admission to the
EMU, J.L. was having 4-6 generalized tonic-clonic seizures per
year, with increased frequency during periods of stress, and daily
morning myoclonic jerks and myoclonic absences.
Patient 2. S.W. is a 47 year-old woman, whose seizure
disorder commenced during early childhood, consisting of
generalized tonic-clonic seizures and occasional staring spells.
Her mother had rubella during pregnancy, and S.W. was born with
significant hearing deficits requiring hearing aids. There is a
questionable family history of epilepsy in a sister. S.W. was
treated with phenytoin and phenobarbital over many years and had a
brief trial of valproic acid, which reportedly caused significant
moodiness and resulted in its discontinuation. Eventually, she was
switched to carbamazepine prior to being monitored in the EMU. She
was having 10-20 generalized tonic-clonic seizures per year
and up to 2-3 staring spells per day. During her hospital
admission, a brain MRI revealed some non-specific white matter
changes suggestive of microangiopathic disease but was otherwise
normal. Neuropsychological testing revealed no lateralizing
abnormalities.
Patient 3. M.M. is a 34 year-old woman with a 19-year
history of generalized tonic-clonic seizures. She also had other
spells’ consisting of subjective dizziness and a sensation of
choking, peri-orbital swelling, and limb shaking lasting for
30-40 minutes. Over many years she had been tried on multiple
AEDs, and was taking carbamazepine, phenobarbital, topiramate, and
clobazam on admission to the EMU. She was having up to one to two
generalized seizures and several other “spells” weekly. In
hospital, brain MRI was normal and neuropsychological testing
showed above average intellect and no lateralizing deficits.
Results
Interictal EEG showed very active bilaterally synchronous
generalized spike-and-wave or polyspike-and-slow wave discharges
between 2.5-4.5 Hz, maximal over the midfrontal structures
symmetrically in all patients with no evidence of lateralized
focality (figures 1,
2, and 3). Coherence analyses [6]
of the spike-and-wave activity showed no hemispheric time leads
(data not shown). Recorded clinical seizures in these three
patients all demonstrated definite, sustained (10-15 seconds),
lateralized clinical features at onset.
Patient 1. Video-EEG revealed one generalized tonic-clonic
seizure, the culmination of increasingly frequent, symmetric, early
morning myoclonic jerks and myoclonic absences. The generalized
tonic-clonic seizure commenced with a sustained forced head and eye
version to the left with extension of the left arm, followed by a
symmetric tonic phase and then bilateral clonic activity
(videosequence 1).
The EEG demonstrated increasing bursts of generalized
spike-and-wave and polyspike-and-wave activity leading up to the
ictal event, which consisted of generalized, initially 3 Hz
bilaterally synchronous and symmetric slow wave activity, which
increased in amplitude and frequency to 3-5 Hz, and then was
obscured by muscle artifact. Subsequently, the recording showed a
clonic de-recruiting response associated with clinical clonic
seizure activity, followed by ictal offset (figure 4).
A diagnosis of juvenile myoclonic epilepsy (JME) was made, and
J.L. was changed from carbamazepine to valproic acid with complete
cessation of his seizures and morning myoclonus, now with over
18 months follow-up.
Patient 2. Four separate clinical seizures were recorded.
The first was a typical absence seizure lasting several seconds.
The other seizures began with a definite head/eye/torso version to
the right followed by a cry and subsequent generalized tonic-clonic
motor activity (videosequence 2).
The EEG recordings during the clinically lateralized tonic-clonic
seizures showed ictal activity consisting of initially 3 Hz
spike-and-wave activity that merged into lower amplitude faster
activity and which ended with a clonic de-recruiting response
(figure 5).
A diagnosis of PGE was made, and S.W. was changed to lamotrigine
and phenobarbital with over 90 % seizure reduction. S.W. has
not agreed to retry valproic acid therapy.
Patient 3. M.M. had one prolonged non-epileptic episode and
two clinical epileptic seizures. The non-epileptic event lasted
twenty minutes and entailed hyperventilation, followed by trembling
of the face and body and finally an arrhythmic shaking of all
extremities. The features fluctuated over time, and she was able to
respond appropriately to questions from her nurse during the
event.
The clinical seizures both commenced with a forced head version to
the left followed by a leftward fencing posture. The seizures
evolved into generalized tonic-clonic motor activity, which at
offset were briefly asymmetric involving unilateral clonic
movements of the right face and arm (videosequence 3).
No EEG changes were recorded in association with the non-epileptic
event. The ictal EEG for the epileptic seizures showed a
generalized, bilaterally synchronous and symmetric 4.5 Hz
spike-and-wave activity with a bifrontal maximum. The EEG evolved
into 4 Hz sharp theta wave activity before becoming obscured
by muscle artifact, though at times an 8 Hz rhythmic epileptic
discharge was visible (figure 6).
A final diagnosis of PGE was made (as well as non-epileptic events
to account for her prolonged “spells”) and M.M. was taken off all
of her admission medications except phenobarbital and was started
on valproic acid. She has remained seizure-free (including her
non-epileptic “spells”) for over one and a half years.
Discussion
Generalized tonic-clonic seizures in primary generalized
epilepsy (PGE) are presumed to commence clinically and
electrographically in a bilaterally synchronous and symmetric
fashion. The pathophysiologic substrate for these seizures includes
reciprocal connections between thalamic and cortical neurons;
connections that are normally responsible for oscillating rhythms
including sleep spindles [1]. At the cellular level, alterations in
receptor function in the thalamic nuclei have been postulated to be
involved in the generation of abnormal synchronized rhythmic
discharges [7] that correlate clinically with generalized seizures
in animal models of PGE. To elucidate whether the initial rhythmic
discharges of a generalized ictal electrographic event arise from
the thalamus, several studies using depth electrode recordings
within various thalamic nuclei compared with surface EEG recordings
have resulted in conflicting results. In 1941, Penfield proposed a
“centrencephalic” hypothesis: using surface EEG recordings with
nasopharyngeal electrodes, Penfield and Jasper concluded that the
thalamus was the focus of “grand mal” (tonic-clonic) seizures based
on phase reversal of epileptiform discharges at the nasopharyngeal
electrodes [8]. Other studies have refuted this hypothesis, in
animal models of feline penicillin generalized epilepsy [9, 10] and
in human depth electrode studies [11], showing evidence for
cortical initiation of generalized seizures. At present, no
definitive conclusion can be drawn as to the anatomical and
physiological origin of generalized seizures in PGE though clearly
an integral role exists for the reciprocal connections within the
cortico-thalamo-cortical network in seizure propagation and
maintenance [1].
Some clinical reports have provided evidence contrary to the
postulated generalized onset of seizures in PGE, with either
lateralized clinical features or with EEG asymmetries [3-5].
Lateralized clinical features are very common in partial
epilepsies. Forced head version prior to generalization lateralizes
to the contralateral hemisphere [12] and is seen in both frontal
lobe seizures and temporal lobe seizures, the latter likely a
manifestation of seizure spread to frontal lobe structures. Other
well-defined lateralizing features include ictal fencing posture
[13], asymmetrical tonic limb posture [14], contralateral ictal
dystonia [15], postictal nose rubbing [16, 17], and asymmetrical
terminating clonic jerks [18]. Focal features described in the
context of PGE are rare, though reports of circling or versive
seizures [19, 20], hemiconvulsive seizures [3] and seizures with
lateralized onset [5] have provided evidence to support a focal
seizure generator. Lateralized findings in JME are more commonplace
and both clinical, specifically asymmetric myoclonus, and EEG
asymmetries can be noted in up to 30 % of patients in some
series [4].
We show here six seizures with definite clinical lateralization in
three patients in whom a confident diagnosis of JME (one patient)
or PGE (two patients) was made. Our patients have no focal
structural, neuropsychological, or electrographic abnormalities to
account for the lateralized clinical onset of tonic-clonic seizures
and they meet all criteria (including clinical and EEG features,
family history, and response to appropriate AED) for a diagnosis of
PGE. However, all of these seizures commenced with forced
head/eye/torso version or a tonic fencing posture clinically
indistinguishable from frontal lobe seizures. These features
support the existence of a focal cortical generator, plus or minus
a lateralized propagation preference, situated in the frontal lobes
of such patients with PGE who present with stereotyped
lateralization at onset of clinical seizures, which may represent a
region of functional hyperexcitability difficult to elucidate with
current electrophysiological or imaging techniques. A recent study
using focal magnetic transcranial stimulation demonstrated
asymmetries in the motor thresholds in patients with PGE and
versive or circling seizures, although a consistent lateralization
was not found [21]. It would be interesting to know if ictal SPECT
or interictal PET studies, unavailable in our patients, could
demonstrate localized frontal lobe functional abnormalities in such
patients. Newer techniques including transcranial magnetic
stimulation, functional MRI with simultaneous EEG recording, and
sophisticated models of source imaging with magnetoencephalography
combined with MRI may further clarify the unresolved questions
regarding the origin of primary generalized seizures.
Future efforts to better understand the underlying neuroanatomical
and pathophysiological nature of the generator(s) of primary
generalized seizures may benefit from a focus on patients with PGE
who demonstrate clinical features of lateralized seizure onset
demonstrated with video-EEG monitoring. Based on previous evidence
and our findings, the hypothesis of a hyperexcitable focal cortical
generator in some patients with PGE appears feasible. Whether this
cortical generator is truly autonomous or, alternatively, is
“triggered” by ascending, possibly normal, thalamocortical volleys
remains unresolved. o
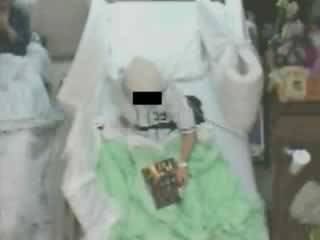
 Video-EEG caption Video-EEG caption |
|
|---|---|
| Time sequence | Clinical features |
| J.L. | |
| 00:00 | Awake; lying supine on bed |
| 00:09 | Start of bilateral limb myoclonus, appearing every 5-15 sec; then increasing myoclonic jerks at 03:54 |
| 04:15 | Raises right arm to adjust (lower) head of bed; ongoing myoclonic jerks |
| 04:30 | Seizure onset with left arm extension and forced head deviation to left; then generalized high amplitude limb myoclonus |
| 04:48 | Tonic extension of all limbs; then bilateral symmetric clonic movements of the extremities at 05:18 |
| 05:37 | End of clinical seizure |
| 05:50 | End of video segment |
| S.W. | |
| 00:00 | Awake; sitting on bed holding a book and conversing with her mother |
| 00:13 | Looking to the right, stops talking and moving; mother still talking |
| 00:19 | Mother asks, “...you know that, eh ?” with no response |
| 00:25 | Sitting looking to the right, unresponsive; mother calls, “Susan ?” |
| 00:28 | Forced right head version and then sustained torso deviation to the right |
| 00:39 | Start of epileptic cry |
| 00:47 | Falls into bed sheets face down with only small movements in limbs |
| 01:25 | End of clinical seizure; starts to get up using left arm and settles into bed |
| **** | |
| 01:48 | Start of second segment; lying supine in bed with nurse at bedside |
| 01:52 | Second seizure onset with forced head deviation to the right |
| 02:02 | Epileptic cry with sustained head and then torso deviation to the right |
| 02:18 | Head turning toward midline and lifting right arm |
| 02:20 | End of video segment |
| M.M. | |
| 00:00 | Awake; sitting in bed looking to the right (watching television) |
| 00:11 | Forced head and then torso deviation to the left; left arm flexed and raised in the air with notable fine shaking movements |
| 00:23 | Leftward fencing posture (left arm tonic extension and right arm flexed to right above head), then clonic jerks of right arm |
| 00:40 | Tonic extension of all extremities followed by bilateral clonic jerks |
| 01:11 | End of clinical seizure with final clonic jerk in right arm |
| 01:25 | End of video segment |