Epileptic Disorders
MENUTwo related cases of super-refractory status epilepticus due to 4-aminopyridine intoxication and an overview of the literature Volume 23, numéro 3, June 2021
An 80-year-old Caucasian woman in status epilepticus was brought to our emergency room (ER). She had no history of epilepsy or any other neurological disorders. Despite treatment with benzodiazepines, levetiracetam and propofol, EEG continually showed generalised epileptic discharges. Only after administering phenobarbital was burst-suppression achieved. Initial work-up revealed no underlying cause.
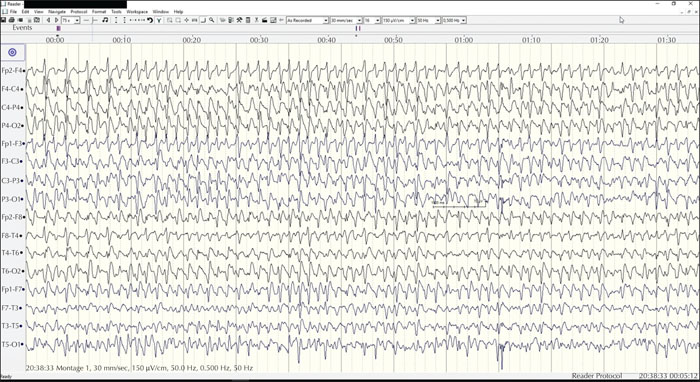
Four days later, her 85-year-old husband arrived at the ER. He had called the family physician because he was feeling unwell after taking medication at home. He was found unconscious in status epilepticus and brought to the hospital. He received benzodiazepines and second-line treatment with levetiracetam and valproic acid. Because of refractory seizures and biochemically severe lactic acidosis, he was sedated and intubated. Seizures remained refractory on EEG despite propofol and midazolam (figure 1). Only after administration of phenytoin and phenobarbital was the status epilepticus interrupted, after four hours.
The medication the husband had taken was a pharmaceutical preparation made by his wife, prescribed for her myalgia and muscle cramps. According to the label, the medication contained 25 mg quinine sulphate, 162.5 mg amidopyrine, 187.5 mg phenazone, and 150 mg acetylsalicylic acid. Toxicological analysis, however, showed a strong positive result for 4-aminopyridine (4-AP). Revision by the pharmacist who was responsible for issuing the prescription confirmed the mistake: a container with 4-aminopyridine had been used accidentally instead of amidopyrine. We suspect the male patient shared his wife's medication as he considered it to be merely a painkiller.
Both were managed in the ICU department under continuous EEG monitoring. The female patient responded well, and after tapering sedation, she improved neurologically and carried out simple tasks. All anti-epileptic drugs (AEDs) could be tapered and she was transferred to the geriatric department. Physically, she recovered, but her cognitive status remained poor with an MMSE score of 21/30. After a few months of rehabilitation, she was discharged to a nursing home facility.
Her husband remained in a non-convulsive status for an entire week despite treatment with multiple AEDs (levetiracetam, valproic acid, phenytoin, phenobarbital, topiramate and lacosamide). Eventually AEDs could also be tapered and he was extubated. Clinically, however, he remained unresponsive. Neither CT nor MRI brain showed any specific abnormalities. After 10 weeks without improvement, a palliative treatment was started and the patient died. This discrepancy in outcome is most likely due to the difference in plasma concentration of 4-AP, as determined by toxicology (table 1). It was later reconstructed that the female patient probably took only one tablet, while her husband had most likely taken two.
4-AP is well known for its epileptogenic properties. It was originally developed for the farming industry as an avicide. Upon ingestion, birds experience a generalized seizure and emit a distress call which scares away other birds [1].
4-AP is a voltage-gated potassium channel blocker which prolongs action potentials by inhibition of potassium ion current causing repolarisation. It also enhances calcium influx and synaptic transmission by increasing acetylcholine release. This results in hyperexcitability of the central nervous system [2-4].
In demyelinating disorders, it has shown to improve axonal and impulse conduction, presumably by the blockade of potassium exchange on the exposed channels of demyelinated axons. For this reason, it is used to improve ambulation in MS patients [1, 2]. It is recommended to perform an EEG to exclude epileptic activity before starting treatment with 4-AP.
4-AP toxicity leads to a sequence of symptoms: hypersalivation, sweating, tremor, epileptic convulsions and cardiac arrhythmia [1, 5]. The therapeutic range of 4-AP is 25-75 ng/mL in serum, and toxicity occurs from 150 ng/mL upwards. Despite this rather narrow therapeutic window, when one keeps to the recommended dose of a maximum of 10 mg, twice daily, preferably in a slow-release form, adverse events are uncommon or mild [2]. One study showed that seizures start to occur from 0.8 mg/kg body weight. It was also determined that within the therapeutic range, the risk of seizures is no higher in a patient group taking 4-AP than in a control MS group [4, 6, 7].
4-AP is eliminated almost exclusively by glomerular filtration. Patients with renal impairment have a higher peak serum concentration and lower clearance rate. While the half-life of the drug lies between 5-24 hours, it is important to be aware that 4-AP-induced encephalopathy may last for several days [2, 8].
A summary of published case reports of 4-AP intoxication leading to epileptic seizures or status epilepticus is presented in table 1. Most of the patients described in the literature developed their first symptoms very quickly after ingestion of the medication, often starting with abdominal discomfort, diaphoresis and agitation, followed by epileptic seizures.
The first step in treating 4-AP overdose is to prevent further absorption by active charcoal administration. This requires a quick intervention (within an hour of ingestion). As with every epileptic seizure threatening to develop into status, after stabilizing the patient, the first priority is to administrate benzodiazepines immediately. Its GABA-ergic mechanism may antagonise the electrical effect caused by 4-AP. Beyond this, there is no specific preference amongst AEDs in cases of 4-AP-induced epileptic seizures. Some suggest a preference for the use of sodium blockers hypothesizing a more effective inhibition of the action potential propagation and subsequently stopping the spreading of epileptic activity. Both our patients received several different AEDs, but eventually required high doses of phenobarbital to terminate the status epilepticus.
In other refractory SE syndromes, such as those caused by nerve toxins, good results have been obtained by the use of ketamine and atropine to terminate SE as a competitive antagonist of acethylcholine on muscarinic receptors [9].
In the available literature, there is a broad range of reported toxicity levels. Five cases were caused by human mistakes: three by prescription errors affecting a total of six patients, and three by (unintentional) incorrect usage by the patients for whom it had been prescribed. Another two cases were caused by intentional incorrect usage (overdose and illegal usage). Clearly the bulk of the problem is due to human error, and even when every precaution is taken by the physician and pharmacist, we must accept ‘Errare humanum est’.
Supplementary material
Summary slides accompanying the manuscript are available at www.epilepticdisorders.com.
Disclosures
There are no disclosures to report.