Epileptic Disorders
MENUTrue abdominal epilepsy is clonic jerking of the abdominal musculature Volume 22, numéro 5, October 2020
Neurogenic causes are rarely considered in the differential diagnosis of patients with episodic abdominal complaints (Stevens, 1949). Abdominal epilepsy (AE), first termed by Brams (1922), is reported to be an exceptionally rare epilepsy syndrome in children and adults (WebMD, 2019) associated with paroxysmal gastrointestinal sensory symptoms including pain as an epileptic equivalent (Moore, 1945; Hoefer et al., 1951). The basis for a diagnosis of AE included a variety of symptoms involving the abdomen or central nervous system, EEG abnormalities and a clinical response to antiseizure medication (Dutta et al., 2007; Mondal et al., 2014). Some diagnoses of AE described neurological involvement including epileptic seizures, though symptoms such as fever (Naeye, 1958), vegetative signs (Baust et al., 1971), and other vague symptoms were classified as neurological evidence of AE (Zinkin and Peppercorn, 2005). Gastrointestinal symptoms varied and include episodic abdominal pain, but also nausea, diarrhea and cyclical vomiting (Mitchell et al., 1983; Peppercorn and Herzog; 1989; Scotiniotis et al., 2000). Abdominal pain is a central feature to the semiology of abdominal epilepsy in the majority of patients with heterogenous onset, intensity, and location (Zinkin and Peppercorn, 2005). Inter- and intra-patient variability of frequency, intensity and duration of abdominal complaints ranged from seconds to several hours (Zarling, 1984). Some patients with AE were later found to have an alternate non-epileptic source (Drexler et al., 1989). Newer terminology such as “focal epilepsy with ictal abdominal pain” has been proposed to more closely represent abdominal epilepsy (Cerminara et al., 2013). To this end, we describe a series of three patients with unequivocal evidence of motor signs associated with focal epilepsy, manifesting as prolonged isolated abdominal clonic jerking confirmed by video-EEG monitoring.
Methods
We searched the Medline database for the literature on abdominal epilepsy limited to human studies and located 139 articles via a library-based literature review using the search terms: “focal seizures AND abdomen”, “epilepsy AND abdomen”, and “seizures AND abdominal epilepsy”. Many of the publications exist in a foreign language but were reviewed if they had an English abstract. Terminology included “truncal” and “axial” seizures for cross-referencing.
Results
The literature search on AE yielded six series involving 47 cases of abdominal epilepsy reported in the American literature, representing the work of 13 authors. There were 36 cases reported in medical journals in the last 40 years. Five series covered 19 cases in international journals reported in English detailing case reports of AE, and 22 case studies were reported in both American and International journals. Duplicate case studies, single case reports and case series, as well as reviews on AE documented variable seizure types and descriptions, inconsistent interictal EEG findings, and a variable response to antiseizure medication (table 1table 2) (Moore, 1945; Moore, 1946; Stevens, 1949; Livingston, 1951; Douglas and White, 1971; Peppercorn et al., 1978; Young and Blume, 1983; Peppercorn and Herzog, 1989; Siegel et al., 1999; Dutta et al., 2007; Tiamkao et al., 2011; Mondal et al., 2014).
Representative cases
Case 1
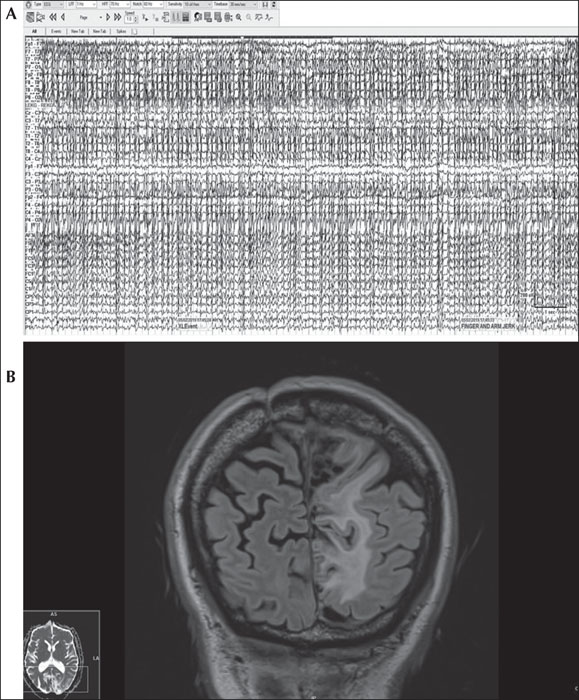
A 48-year-old woman with hypertension and hyperthyroidism had a three-year history of spells consisting of stereotyped, rhythmic abdominal clonic movements. The patient described “jumping in my stomach” and rare sensations of subjective bilateral leg trembling that travelled up her arms. Her family history was notable for “grand mal” seizures in a sister, son, and maternal niece. Brain MRI revealed venous lakes in the calvarium, mild chronic microvascular ischemic changes, and an arachnoid cyst along the right frontoparietal lobe splaying the central sulcus. Increasing frequency of the episodes resulted in several emergency department visits despite an empiric trial of levetiracetam, 2,000 mg daily, and lorazepam, 1 mg twice daily. Multiple interictal EEGs remained normal. She was admitted for diagnostic video-EEG monitoring and differential diagnosis of recurrent spells. Levetiracetam was discontinued and she experienced multiple events of her “tummy jumping” in addition to feeling the need to have a bowel movement. Bilateral clonic jerking of the abdomen was palpable on abdominal inspection with rare low-amplitude involvement of her right, more than left, mesial thighs. Off antiseizure medication, the involuntary episodes intensified and prolonged to nearly one hour of continuous motor jerking at a frequency of 2 Hz. Video-EEG revealed diffuse, periodic repetitive myogenic artifact during seizures in sync with clonic jerking of the abdomen (figure 1A). During this time, she was tachycardic, hypertensive, and diaphoretic but alert, responsive, and able to follow commands. The patient progressively fatigued and became exhausted from the event despite continuing to verbalize and respond to questioning. She and her husband became increasingly agitated with systolic blood pressure reaching 200 mm Hg, resulting in administration of 2-mg intravenous lorazepam followed by immediate (<5 min) and complete cessation, and subsequently severe postictal exhaustion and generalized weakness. Post-ictal EEG revealed left temporal 4.5 to 5.5-Hz theta slowing. Intravenous loading with valproate and conversion to oral valproate resulted in sustained seizure freedom.
Case 2
A 67-year-old man with a recurrent atypical meningioma and drug-resistant focal epilepsy had a 10-year seizure history of generalized tonic-clonic seizures. Despite control of convulsions, he took four antiseizure medications and continued to experience episodes of tingling in his right foot that would march up his leg to involve the right side of his abdomen prior to isolated episodes of abdominal twitching twice weekly. The movements, at times, would be so severe that his entire trunk would move in a compensatory fashion due to the greater intensity of abdominal twitching. With intensification, subjective sensory symptoms would spread to involve his face and right arm despite isolated motor manifestations in his abdomen and retained awareness. Events were occasionally triggered by somatosensory stimulation. Repeat hospitalizations were required for prolonged abdominal jerking. Brain MRI demonstrated left parietal encephalomalacia from prior craniotomy for tumor resection (figure 1B). Standard EEGs were unrevealing. He was admitted for video-EEG monitoring and with scalp EEG placement of the 10-20 system; EEG was normal during events of abdominal twitching. When high-density EEG using the 10-10 system with extra parasagittal electrodes was used, EEG was abnormal with frequent spikes in the left central parietal lobe (CP5, P1, P3, maximal at P5). Focal subclinical seizures also arose from the left central parietal region (CP5, P5), and focal aware motor seizures manifesting as abdominal twitching arose from the posterior frontoparietal high parasagittal region (C1-FC1). After taper of lamotrigine and levetiracetam, video-EEG monitoring captured an electroclinical focal impaired awareness seizure. The patient reported “something is coming on” with a sensation in his abdomen and right testicle, evolving to impaired awareness and clonic jerking isolated to the abdomen (see video sequence), with intensification of motor symptoms progressing to clonic jerking of his right shoulder and “march” to affect his right arm with flexion tonic posturing and extensor tonic stiffening of his right leg, followed by right facial clonic jerking lasting for seven minutes. Neuromodulation was recommended at surgical epilepsy conference but was deferred.
Case 3
A 55-year-old right-handed woman with hypertension had a six-month history of recurrent spells. An initial episode of nausea and vomiting followed by right arm and leg numbness lasted for five minutes until she lost consciousness. The Emergency Medical System was activated, and she was taken to the nearest local hospital. Upon evaluation, “seizure-like activity” was observed. Lorazepam and fosphenytoin were administered. In retrospect, she reported similar symptoms since January 2017, manifesting as episodes of light “tickling” over the sole of her right foot, lasting for 1-2 minutes. Brain CT demonstrated a densely calcified extra-axial mass in the left parietal region with a small amount of associated vasogenic edema. Her neurological examination was normal. Brain MRI revealed two enhancing lesions within the left paramedian frontoparietal lobe and a small one in the left frontal lobe. EEG demonstrated continuous left hemispheric slowing, maximal in the left parieto-occipital region. She subsequently underwent a craniotomy and resection of the lesions with minimal residual tumor surrounding the superior sagittal sinus. She completed a course of radiotherapy.
Following radiotherapy, she developed a new type of brief, stereotyped event involving her right foot two months later, manifesting as a sudden, intense tingling in her toes (especially the great toe), followed by “weakness or numbness” that usually occurred during walking leading to her stumbling. She would then have rhythmic muscle “squeezing” that marched up her right leg to the knee. Over time, contractions progressed from her leg to the thigh, hip, and abdomen, rarely spreading to the small of her back but without arm or face involvement, and without alteration of awareness, 1-2 times per month. She then underwent inpatient video-EEG monitoring with three focal seizures recorded on EEG. The last seizure started with subjective right toe tingling, progressing to complaints of muscle clenching of the right leg and then objective marked abdominal clonic twitching. During this time, she was awake, alert, speaking, and following instructions throughout. This last instance was associated with an electrographic correlate occurring 40 minutes following clinical onset.
Discussion
To the best of our knowledge, our series of patients with lesional extratemporal focal epilepsy is the largest series of focal aware motor seizures isolated to the abdominal musculature as the primary semiology confirmed by video-EEG monitoring. Seizures consisting of isolated truncal muscular contraction involving the abdomen have rarely been reported (Matsuo, 1984; Rosenbaum and Rowan, 1990; Aljaafari et al., 2018). Focal aware motor seizures involving the abdominal musculature are at odds with prior reports of AE involving abdominal pain in pediatric patients (Livingston and Schwentker, 1950; Devore et al., 1955; Peppercorn et al., 1978; Peppercorn and Herzog; 1989). A recent case report by Aljaafari and colleagues (2018) reported a 26-year-old man with unilateral abdominal clonic seizures similar to our second case. In contrast, our series contains lesional epilepsy patients with prolonged seizures and epilepsia partialis continua to add to 13 prior cases reporting patients with persistent myoclonus or jerking of the abdominal musculature (Asranna et al., 2019). In the prior review on abdominal epilepsia partialis continua, nearly a half of the cases were without EEG confirmation (due to a normal, unavailable, or unreported EEG) during the episodes, myoclonus was involved as the semiology in some patients, and a series of clonic EPC validated by video-EEG monitoring was not reported.
Abdominal symptoms as seizures
Abdominal auras are common focal aware seizures present in patients with mesial temporal lobe epilepsy (Tatum, 2012). In a retrospective study of 223 patients with abdominal auras (e.g., rising epigastric auras, borborygmus, nausea), temporal lobe epilepsy was more frequent than extratemporal epilepsy, and mesial was more frequent than neocortical temporal lobe localization without preferential lateralization (Henkel et al., 2002). Older series reported only two of 30 patients with isolated pain as an ictal correlate (Mauguiere and Courjon, 1978). In more recent series, abdominal pain as an aura was found to represent 5% of patients with temporal lobe epilepsy and 50% with frontal lobe epilepsy (Nair et al., 2001). All our patients had extratemporal epilepsy in line with other reports involving seizures and the abdominal musculature (Matsuo, 1984; Rosenbaum and Rowan, 1990; Nair et al., 2001; Aljaafari et al., 2018; Asranna et al., 2019).
Migraine overlap has been postulated as one mechanism to explain episodic abdominal symptoms in patients with epilepsy (Moore, 1950; Reimann, 1973). A large retrospective study of 100 epilepsy patients, 100 migraine patients and 100 controls reported periodic symptoms of abdominal pain that were significantly more frequent in children with migraine (Lanzi et al., 1983). In 1902, Jackson and Singer postulated that the origin of truncal seizures is localized within the brainstem when patients experienced bilateral seizures of the face and abdomen with loss of consciousness (Oster et al., 2011). Hypermobility of the bowel provoked by “abnormal discharges of neurons in the vicinity of altered cerebral tissue” was presumed to reside in the pre-motor or post-motor cerebral cortex and/or diencephalon (Moore, 1945; Moore, 1962). Four patients with “axial” seizures confined to the face, tongue, palate, pharynx, diaphragm, and abdomen were previously reported using 16-mm motion pictures and EEG, suggesting a brainstem origin (Nathanson et al., 1978). EEG during midline seizures displayed “periods of burst activity followed by relative interictal electrocerebral silence”, reminiscent of our first case obscured by rhythmic artifact from clonic jerking (Nathanson et al., 1978). Later, anatomic connection of the amygdala and/or hypothalamus linking the gut via the vagus nerve and sympathetic nervous system was postulated as the pathophysiology for abdominal epilepsy (Zinkin and Peppercorn, 2005). Thalamic connections to peripheral structures involved in pain perception were also felt to play a role (Montavont et al., 2015). Currently, the parietal operculo-insular cortex is felt to play a principal role in the sensory-discriminative processing of pain input with high value for seizure localization and lateralizion to the contralateral hemisphere in patients with focal seizures (Montavont et al., 2015). Van Buren (1963) reproduced abdominal symptoms using direct electrical stimulation of the brain in epilepsy patients implanted with limbic depth electrodes. On the contrary, seizures in patients reported with abdominal epilepsy have also been reproduced by intestinal stimulation, raising alternative possible mechanisms for seizure genesis (Gastaut and Poirier, 1964).
Video-EEG monitoring
Previously, the diagnosis of AE was based upon an abnormal EEG (Devore et al., 1955), including epileptiform discharges (Papatheophilou et al., 1972; Garcia-Herrero et al., 1998) and “dysrhythmias” (Bental et al., 1967), with only a few reports on ictal EEG (Matsuo, 1984; Cerminara et al., 2013; Aljaafari et al., 2018). Previously, studies on patients diagnosed with AE focused on temporal epileptiform abnormalities (Moore, 1962; Zinkin and Peppercorn, 2005). Discharges localized to the parietal lobe were reported infrequently (Zinkin and Peppercorn, 2005), though frontal (Lim et al., 2004), and perisylvian locations have also been noted (Garcia-Herrero et al., 1998). Nevertheless, interictal epileptiform discharges are not confirmatory unless ictal recordings are obtained (Tatum et al., 2018). In prior case reports involving a diagnosis of AE linked to interictal EEG, generalized spike-and-wave was reported (Yunus et al., 2016), though this epileptiform abnormality is unlikely to cause abdominal pain. In addition, published EEG samples, as the basis for diagnosis of AE in other reports, appear to be misinterpreted as normal physiological (drowsy) bursts and artifact (Dutta et al., 2007). Olmez et al. (2006) described a patient with cyclic vomiting and generalized epileptiform discharges on EEG who responded to topiramate but pointed out that cyclical vomiting has a migrainoid basis for which treatment with topiramate would be explanatory. Rarely, electrographic seizures have been identified as the substrate for intense abdominal pain (Gastaut and Poirier, 1964) and unilateral abdominal clonic jerking (Aljaafari et al., 2018). Rare accounts of abdominal epilepsia partialis continua throughout the world demonstrate significant variability in semiology, EEG features, and underlying pathology (Asranna et al., 2019).
In our series, video-EEG monitoring was required to validate the diagnosis of focal aware motor seizures after interictal EEG was non-epileptiform. Rhythmic myogenic artifact followed by postictal slowing, epileptiform activity only following application of high-density electrodes, and an ictal EEG underscore the variability of EEG findings and the need for video-EEG monitoring to verify the epileptic origin. Prolonged seizures including abdominal epilepsia partialis continua were common in our series and resulted in fatiguability and systemic adverse effects during prolonged episodes, underscoring the need for prompt treatment to prevent medical consequences. Non-convulsive status epilepticus associated with AE was previously reported in a patient from Thailand, albeit with limited detail (Tiamkao et al., 2011).
Treatment
Episodic abdominal pain should exclude intra-abdominal causes when separating symptoms of abdominal epilepsy from a surgical abdominal (Moore, 1950) or comorbid abdominal pathology, though many are diagnosed as functional (Topno et al., 2005). Prior reports of appendectomy for unconfirmed appendicitis (Stevens, 1949) and spinal cord tumors mimicking abdominal epilepsy have been reported (Drexler et al., 1989). In contrast to prior reports, our case series was comprised of patients with drug-resistant focal epilepsy confirmed by ictal EEG during video-EEG monitoring. Transient brain MRI (Dafotakis et al., 2006) and functional neuroimaging with single-photon emission computed tomography have been used to substantiate truncal seizures (Oster et al., 2011), though our patients had concordant abnormal baseline anatomic imaging. The ability to obtain a definitive diagnosis with video-EEG monitoring has forced a reappraisal to classify epilepsy syndromes and impact treatment (Douglas and White, 1971). Our patients with AE (Siegel et al., 1999) presented with similar electro-clinical features supporting a contralateral parietal seizure onset zone arising from the trunk area of the sensory homunculus in the postcentral gyrus (Moore, 1962). Focal aware seizures involving the abdominal musculature continue to be rare events (Matsuo, 1984; Rosenbaum and Rowan, 1990; Aljaafari et al., 2018) probably due to a relatively high seizure threshold in the area of brain sub-serving the abdomen and truncal area, the small anatomic representation, and limitations of standard EEG to recover an abnormality which poses diagnostic challenges. Reports of myoclonus as epilepsia partialis continua (Lim et al., 2004; Tezer et al., 2008; Asranna et al., 2019), other concomitant seizures types (Matsuo, 1984), seizure involvement outside the abdomen (Lim et al., 2004; Tezer et al., 2008), and a lack of video-EEG confirmation underscore the immediate need to update the syndrome of AE using current terminology approved by the International League Against Epilepsy (Scheffer et al., 2017).
Conclusions
The diagnosis of AE has previously been based on rare case reports and small series. The validity of non-motor seizures reported as AE has been questionable at best with multiple confounding factors. Focal aware motor seizures involving clonic jerking of the abdominal musculature in our series truly reflect AE as the principal semiology in patients with focal epilepsy confirmed by video-EEG monitoring. In our patients, AE was localized to the centroparietal midline involving the truncal area of the sensorimotor homunculus by neuroimaging. We recommend abandoning usage of the term “abdominal epilepsy”, and replacing it with the new terminology recognized by the International League Against Epilepsy as “focal aware motor seizures with abdominal clonic jerking”, in addition to confirmation of an ictal origin with video-EEG monitoring to validate the diagnosis.
Supplementary data
Summary didactic slides are available on the www.epilepticdisorders.com website.
Acknowledgements and disclosures
We thank Mayo Clinic Scientific Publications for academic assistance formatting and submitting this paper.
None of the authors have any conflict of interest to declare.
* Presented in pat at the American Clinical Neurophysiology Society annual meeting, Las Vegas, NV, February 8, 2019.