Epileptic Disorders
MENUThe varied semiology of seizures in the context of small anterior temporal encephaloceles Volume 21, numéro 4, August 2019
Advances in neuroimaging have resulted in small anterior temporal encephaloceles (TE) being increasingly identified in patients with medically refractory epilepsy (Saavalainen et al., 2015; Panov et al., 2016; Toledano et al., 2016). Several reports have characterised the large majority of seizures as being concordant with a temporal lobe onset (Saavalainen et al., 2015; Panov et al., 2016; Toledano et al., 2016). In the experience of our centre, seizure semiology can vary significantly in these patients and is not always in keeping with a focal aware or focal seizure with impaired awareness and motor automatisms reflective of temporal lobe epilepsy (Saavalainen et al., 2015; Toledano et al., 2016). The following brief report aims to emphasize this point and serves to document that seizures from such encephaloceles can appear extra-temporal. The report goes on to describe the ictal propagation patterns in these patients, recorded with intracranial EEG. There are only limited descriptions in the literature that are components of larger descriptive studies.
Methods
Patients
Patients were selected from the Epilepsy Surgery Database at our facility between December, 2012 and April, 2017. Since 2012, SEEG was utilised to evaluate non-lesional cases of focal epilepsy. The database consisted of 143 patients who underwent epilepsy surgery. Out of these 143 patients, six had temporal encephaloceles (4.2%), four of whom were selected to undergo intracranial EEG assessment before resection. These patients were selected as they had the combination of dominant hemisphere encephaloceles in which the extent of resection was pertinent to neuropsychological outcomes. Importantly, in these four patients, the semiology was discordant with that expected from a seizure arising from the temporal lobe. The additional two patients with non-dominant hemisphere encephaloceles had seizures concordant with a temporal lobe onset and underwent standard right temporal lobectomies, both resulting in seizure freedom (Engel Class Ia).
Surgical assessment
The four selected patients underwent routine pre-surgical evaluation for epilepsy surgery, which included long-term video-EEG monitoring, neuroimaging with volumetric 3T MRI, high-resolution CT, PET imaging, SISCOM, and neuropsychological assessments. Stereotactic planning was achieved by obtaining T1-weighted volumetric sequences with contrast-enhanced angiogram and venogram phases. SEEG exploration was proposed with electrode placement designed to confirm or refute hypotheses generated by the cumulative information gained from non-invasive assessment. SEEG recording was achieved with the placement of intracranial electrodes with multiple contacts (12-18 contacts, diameter 0.8 mm, length 2 mm, 1.5 mm apart), of which the position was confirmed with post-operative MRI.
Results
Population
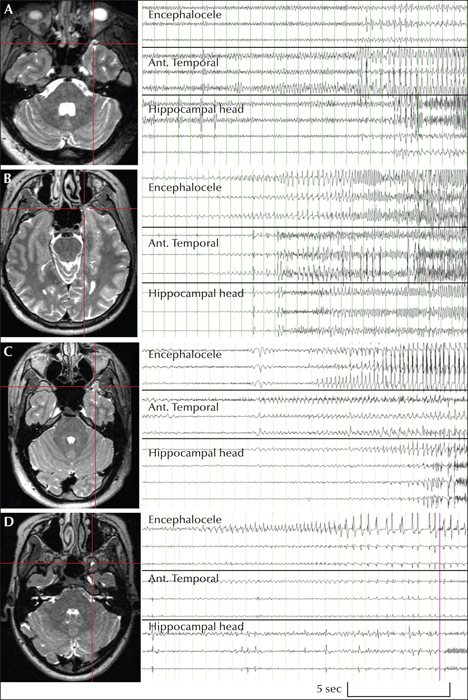
Three males and one female were selected, aged between 20 and 40 years old, with an average duration since epilepsy diagnosis of 10.8 years (range: 3-19). All patients were medically refractory, each having trialled multiple antiepileptic medications and continued to have seizures on a weekly to monthly basis at the time of intracranial assessment. All patients had documented MRI of small TEs of their dominant hemisphere, without the presence of mesial temporal sclerosis (figure 1). Patient 3 had had previous epilepsy surgery in August, 2014 in the temporo-occipital junction following subdural grid assessment, but had no change in seizure semiology or frequency (Engel Class IVb). With the 1.5 T MRI available in 2014, a temporal encephalocoele was not appreciated at the time of subdural electrode evaluation in this patient.
Non-invasive assessment
All patients had focal seizures with impaired awareness. Motor automatisms could be briefly seen but these were not a prominent feature in any of the seizures. The main component of the seizures was hyperkinetic (Patient 1), hemiclonic (Patient 4), or prolonged versive with asymmetric tonic posturing (Patient 2 and 3). The components of the seizure semiology and non-invasive pre-surgical assessment are outlined in table 1. In Patient 2, the localization of the scalp EEG was multiregional, independent of the suggestion of posterior quadrant involvement, a finding that was also seen in Patient 3 who only had epileptiform discharges maximal in the posterior quadrant rather than the expected fronto-temporal region.
Intracranial EEG
Across the four patients, 18 clinical seizures were captured, along with multiple subclinical seizures. Typical clinical and subclinical seizures were all seen to originate from the anterior temporal region from the recording electrode in closest proximity to the temporal encephalocele, with no seizures originating from mesial temporal structures (figure 1). A major finding was that the ictal propagation patterns differed between patients but remained consistent for each patient. In Patients 1 and 2, there was quick spread from the region of the encephalocele and anterior temporal lobe to the head of the hippocampus (< 5sec), whereas Patients 3 and 4 experienced later involvement (> 5sec) (Kahane et al., 2002).
The propagation network consisted of rapid engagement of the orbitofrontal lobe (Patient 1:
Surgical outcomes
All patients had epilepsy surgery with a tailored lesionectomy of the temporal pole. In all the cases, the hippocampus was spared. In all patients, the encephalocele was clearly visible at the time of operation. A critical finding was that all patients demonstrated improved seizure outcomes, irrespective of early or late propagation to the hippocampus or rapid spread to distant brain regions. The patients with early spread into the hippocampus had an Engel Class 1a and 1b outcome after 12 and 24 months of follow-up, respectively. The patients with late involvement of the hippocampus had Engel Class 2d, due to two nocturnal seizures, and Engel Class 1a each after 12 months of follow-up. Histopathology revealed minor layering abnormalities in all the patients along with adjacent gliosis (FCD type 1a: n=1; type 1b: n=1; type 1c: n=2).
Discussion
Temporal encephaloceles, as a cause of refractory focal seizures, has drawn attention in the literature (Saavalainen et al., 2015; Toledano et al., 2016). The current report does not intend to refute the good quality cohort descriptions provided but aims to add to this information. Previous cohort studies have classified the seizures as predominantly concordant with a temporal onset (Saavalainen et al., 2015; Toledano et al., 2016). This is a true statement, however, the current report highlights instances whereby this may be an over simplification of the range of semiology observed in these patients. The report further highlights that there are also cases in which the EEG findings appear discordant with the expected.
The main finding of the report is that even though seizures can appear heterogeneous (both clinically and electrographically), the seizure onset zone was consistently localized to the anterior temporal region. Possible insight into these findings can be gained from interpreting the ictal propagation patterns recorded with intracranial EEG in conjunction with the body of literature describing the connectivity of the anterior temporal lobe.
The connectivity of the anterior temporal lobe has been previously described and the concept of the insula-orbito-polar-temporal network revisited (Chabardès et al., 2002). Ictal spread through this complex likely accounted for some of the clinical findings. For example, Patient 1 had a hyperkinetic seizure type, which coincided with rapid engagement of the orbitofrontal cortex. Hyperkinetic seizures have been described from this location before (Bonini et al., 2014).
The over-representation of fearful emotional content may provide some clues as to limbic/para-limbic involvement. Bonini et al.’s report on sub-division of frontal lobe seizures commented on the combination of high-amplitude hyperkinetic seizures and inappropriately contextualised expression of emotion, being more likely to originate from structures with greater affinity for engaging the limbic system (Bonini et al., 2014). Patients 2 and 3 both with prominent ictal fear coincided with SEEG proven engagement of limbic/paralimbic structures, such as the amygdala and anterior cingulate. Rapid engagement of posterior frontal networks was also seen with Patients 2 and 4, demonstrating forced-eye and head deviation with frontal eye field activation and even hemi-clonic facial jerking with propagation to the motor strip (Patient 4).
Chabardès et al. also outlined the connectivity to the occipito-basal cortex, via the inferior longitudinal fasciculus (Chabardès et al., 2002). This was confirmed in Patient 1. Patient 3 had a previous subdural grid assessment (without recording of the temporal pole), suggestive of a seizure focus in the temporo-occipital junction. This was not replicated, potentially due to disruption of the network from the initial resection. More recent connectivity analysis has documented rapid propagation of seizures from the temporal pole to the posterior language cortex, presumably via the arcuate fasciculus (Ardila et al., 2014). All of our four patients had seizures that propagated to the inferior parietal lobule.
Descriptions of previous invasive assessment of TE seizures have been conflicting. The series by Toledano contained four patients who underwent SEEG and reported seizures originating from the anterior temporal pole, demonstrating “late” involvement of the hippocampus (Toledano et al., 2016). Contrary to this, Panov reported findings in six patients that were assessed with ECoG, followed by subdural grid placement with a depth electrode into the temporal encephalocele (Panov et al., 2016). Two of the patients in this series were noted to have “synchronous or rapid spread” to the hippocampus (Panov et al., 2016).
This variable interaction between the temporal pole and amygdala/hippocampus was previously described by Kahane et al. (2002). They described two groups; one where seizures rapidly (within 5 seconds) spread between temporal polar and mesial temporal structures vs. those that took longer than this. Our cohort confirms that indeed both groups exist. Although the total number of cases is small, even when combined from our study and the literature, it tentatively seems that this feature does not predict surgical failure if a more limited lesionectomy was performed.
Supplementary data
Summary didactic slides are available on the www.epilepticdisorders.com website.
Disclosures
None of the authors have any conflict of interest to declare.