Epileptic Disorders
MENUStereotactic-EEG-guided radiofrequency multiple hippocampal transection (SEEG-guided-RF-MHT) for the treatment of mesial temporal lobe epilepsy: a minimally invasive method for diagnosis and treatment Volume 23, numéro 5, October 2021
Temporal lobectomy is the gold standard treatment for drug-resistant epilepsy arising from the mesial temporal lobe. However, patients who have mesial temporal epilepsy on the language-dominant side with preserved verbal memory are generally not considered good candidates for surgical resection due to a high risk of postoperative memory decline. Several procedures have been developed that minimize the impact on the temporal neocortex, the temporal stem, and even the amygdala-hippocampus itself. For example, it is possible to perform selective amygdalo-hippocampectomy (SAH) using the Hori-technique that approaches the temporal horn from the base of the temporal lobe in order to avoid damage to the temporal neocortex and temporal stem. However, these techniques generally involve complete destruction of the amygdala-hippocampus complex, which is essential for consolidation of memories and therefore still involve substantial risk to verbal memory when performed on the dominant side. Functionally, in a large cohort (n=140) of patients who underwent SAH, 50% of the 66 left SAH patients experienced reliable decline in verbal memory in spite of the potentially protective effect of hippocampal sclerosis in 80% of those patients [1]; these declines persisted for a year after surgery [2].
In order to minimize the risk of postoperative memory decline of patients with dominant mesial temporal lobe epilepsy with high verbal memory scores, our multidisciplinary epilepsy team developed a minimally invasive treatment technique adopting the principles of multiple hippocampal transections (MHT) using SEEG-guided radio frequency thermocoagulations (SEEG-guided-RF-MHT) in order to avoid the discomfort and longer post-operatory recovery time associated with craniotomies.
Multiple hippocampal transections are an alternative to mesial temporal resection. This procedure calls for disconnection of longitudinal hippocampal pathways that allow synchronization of ictal activity and seizure propagation while preserving the transverse lamellae arranged in parallel loops that are associated with memory processing [3]. MHT achieves seizure freedom rates comparable to standard temporal lobectomy [3-5] with preservation of memory in a cohort of patients at high risk of memory decline (Fastenau et al. [abstract] in press, Epilepsia).
In our center, 17 patients with predominantly non-lesional MTLE and high memory scores underwent standard MHT in which the hippocampus was approached in a minimally invasive fashion, either through the middle temporal gyrus or through the Sylvian fissure. Nine patients had a modified procedure that included the removal of the temporal tip, amygdala, and anterior 4.5-cm of the lateral and basal temporal neocortex. The fusiform gyrus was always spared (MHT+). The rest of the cases involved MHT alone with no resection of neocortical brain tissue (MHT-). Overall, seizure freedom was achieved in 64.7% (Engel Class I) at one year [6]. In subsequent analysis of neuropsychological outcomes, one to two years after MHT in our expanded cohort of 22 language-dominant patients with mesial temporal lobe epilepsy (which included the original 17), verbal memory was preserved in 77% of the patients (Fastenau et al. [abstract] in press, Epilepsia).
Although this surgical technique leads to improvement in seizures with preservation of cognitive function, two important limitations have been observed. First, typical approaches to MHT usually produce significant damage to the temporal stem and neocortical temporal structures associated with an approach to the mesial structures. Second, a decrease in hippocampal volumes over the long term, ranging from 17.5% [4] to 25.7% [7], was seen.
Stereotactic EEG-guided radiofrequency thermocoagulation (SEEG-guided RFTC) for the treatment of drug-resistant epilepsy is a minimally invasive technique of epilepsy surgery that has, within the past decade, emerged as a potential method to disrupt epileptogenic networks without surgical resection [8-10]. This approach has been performed for many decades but was initially described in detail by Guenot in 2004 using stereoencephalographic (SEEG) recordings of ictal activity to guide RFTC lesions [9]. Recent literature has highlighted the value of SEEG-guided RFTC when the ictal onset zone is limited in size. SEEG-guided RFTC has also been used when the epileptogenic zone overlaps with highly functional areas and a more extensive resective surgery is not an option [8, 10, 11]. SEEG-guided RFTC is associated with a much lower rate of seizure freedom in comparison to surgical resection or laser ablation, likely related to the relatively small lesions produced by RFTC (5-7 mm diameter of coagulation necrosis around each contact used for ablation), particularly when using only contiguous contacts along a single depth electrode [10-12]. Recently, we observed that bipolar coagulations across contacts of two electrodes placed at up to a distance of 10 mm using lower power for a longer period of time produced larger confluent lesions than traditional parameters described in previous reports where dipoles along a single electrode array were used even with the highest RF current intensity achievable (8 W) [13-15]. Similar observations were reported by Fan et al. to optimally produce complete amygdalo-hippocampal complex ablations in patients with MTLE and hippocampal sclerosis using several stereo-crossed bonding electrodes. The compatibility method was tested between polyacrylamide gel (PAG) and brain tissue and the observed sufficient distance of contacts (from different electrodes) for confluent lesioning was 7 mm with PAG using 3 W over 150 seconds [15, 16]. In this study, the authors performed confluent coagulations to completely ablate the sclerotic amygdala-hippocampal complex. The neuropsychological profile of the cohort was not reported.
In this report, we describe a new strategy of SEEG electrode placement and coagulation that allows the production of linear confluent RF ablations perpendicular to the longitudinal axis of the hippocampus mimicking multiple hippocampal transections, to limit the damage of the amygdala-hippocampal complex, in order to minimize the risk of postoperative memory decline in patients with dominant mesial temporal lobe epilepsy with high verbal memory scores. This report discusses details of the technique, safety of the procedure, and preliminary data on successful seizure control.
Methods
Subjects and settings
We conducted a retrospective case series study for patients with suspected MTLE and high memory scores. Only patients with a high suspicion of MTLE from non-invasive studies with high memory scores were included. The study was approved by our Institutional Review Board.
In a case series of five patients (four females) with a mean age of 39 years (range: 25-54 years) with intractable temporal lobe epilepsy (table 1 table 1), the mean seizure onset age was 21 (range: 19-35 years) and mean epilepsy duration was 17 years (range: 5-40 years). Initial video-EEG monitoring with scalp electrodes revealed unilateral TLE in all but one patient, who had bilateral seizure activity. An intracarotid methohexital sodium injection (WADA) was performed in four of the five patients in whom language dominance was confirmed to be ipsilateral to the seizure focus in three subjects and contralateral in one subject (Subject 2) who declined temporal lobectomy. One subject (Subject 4) did not complete WADA, but this subject was right-handed with left TLE and clear post-ictal aphasia after each seizure. Thus, it was concluded that the dominant hemisphere was ipsilateral to the seizure focus in this case.
All subjects underwent comprehensive neuropsychological testing for visual and verbal memory. Verbal memory and visual-spatial memory were assessed using the Wechsler Memory Scale 3rd Ed. (WMS-III) Auditory Immediate/Delayed Memory Index and Visual Immediate/Delayed memory Index. All four subjects undergoing language dominant RF-MHT had preserved verbal memory presurgically (table 1); the subject undergoing language non-dominant RF-MHT had visual-spatial memory in the borderline range. Invasive monitoring with stereotactic EEG was planned to confirm the localization of the epileptogenic zone. Four patients underwent unilateral depth electrode implantation on the seizure-onset side covering the hippocampus, amygdala, and other structures known to be connected with mesial temporal structures in order to rule out extrahippocampal seizure onset. One patient with suspected bitemporal epilepsy but with greater seizure burden arising from the dominant temporal lobe had bilateral implantation of depth electrodes (Subject 4). Habitual episodes were recorded in all five patients confirming the hippocampi and amygdala as seizure-onset zones.
Based on the neuropsychological scores and non-lesional MRI, it was determined that there would be considerable risk of memory decline if a significant portion of the hippocampus were to be surgically removed. As an alternative to MHT or laser interstitial thermal therapy (LITT), a SEEG-guided-RF-MHT technique was devised. This technique is significantly less invasive than MHT, LITT, selective amygdalo-hippocampectomy, or temporal lobectomy. Also, production of a smaller lesion would not preclude subsequent treatment with more substantial lesioning if seizures were not adequately controlled.
Electrode disposition for transection of mesial temporal structures
Platinium-iridium depth electrodes, measuring 1.1 mm in diameter and 2.5 mm in length, evenly spaced at 5-mm intervals, were implanted stereotactically (Ad-tech Medical, Racine, Wisconsin). Implantation trajectories were simulated using conventional StealthStation software (Medtronic, Minneapolis, MN) based on recent 3 Tesla MRI of the brain (3T Siemens Skyra MRI machine, Siemens, Munich, Germany).
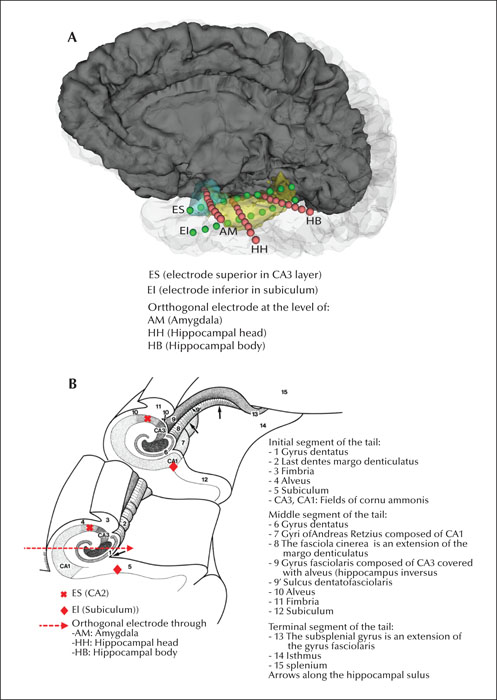
For each patient, four orthogonal electrodes were implanted through the lateral temporal cortex targeting the amygdala (electrode AM), head and body of the hippocampus (electrode HH and HB) and temporal tip (electrode TT). Two additional electrodes were implanted from posterior to anterior, along the longitudinal axis of the hippocampal and entorhinal complex, one superior (electrode ES) to the orthogonal electrodes, and one inferior (electrode EI) targeted to an area between the subiculum, entorhinal area, and inferior longitudinal fasciculus (figure 1 A, B). The ES and EI electrodes were separated by 8-10 mm.
Pre-surgical 3D T1-weighted brain MRI (slice thickness: 0.8 mm; pixel size: 0.8 × 0.8 mm; TR: 2090 ms; TE: 3.24 ms; FA: 15 degrees) was performed using a 3T Siemens Skyra machine (Siemens, Munich, Germany).
Cranial CT (Somatom Definition Flash, Siemens, Munich, Germany) was performed within 24 hours of electrode implantation (slice thickness: 1 mm; pixel size: 0.5 × 0.5 mm). The post-operative CT images were co-registered to pre-operative MRI using affine transformation available in 3D Slicer (www.slicer.org, version 4.8.1). This enabled precise localization of individual electrode contacts in the patient's pre-surgical MRI to plan for ablation.
SEEG-guided radiofrequency transection
SEEG-guided RF-MHT was performed after confirming that the amygdala-hippocampus complex was the epileptogenic zone. During a multidisciplinary conference, a plan for ablations was designed for each case to produce three linear perpendicular ablations in the longitudinal axis of the amygdalo-hippocampal complex with the aim of disconnecting the amygdala from the head of the hippocampus, the head of the hippocampus from the body, and the body of the hippocampus from the tail. These three cuts were performed using adjacent contacts of the same electrodes and contacts of different electrodes.
The RF lesions were performed using an RF generator (Costman RFG-1A, Costman Medical Inc, Burligton, Massachusetts). Alligator clips connected to the active and ground ports were attached to contiguous contacts or contacts on adjacent electrodes. The procedure was performed at the patient's bedside with the patient awake during the entirety of the procedure. A stereotactic neurosurgeon (JM), an EEG technologist, a member of the nursing staff and an epileptologist were always in the patient's room during the SEEG-guided-RF-MHT. Before each ablation, the epileptologist and technologist confirmed the correct placement of the alligator clips for the pair of electrodes included in each coagulation.
We performed bipolar coagulations on three contiguous contacts on each of the orthogonal electrodes at the amygdala, head, and body of the hippocampus. In order to complete three linear lesions perpendicular to the longitudinal axis of the amygdalo-hippocampal complex, we performed bipolar coagulations using one to two cross-bonding contacts of adjacent electrodes at the level of the amygdala (AM to ES and ES-EI), at the level of the head of the hippocampus (ES-HH and HH-EI) and at the level of the body (ES-HB and HB-EI) (figure 1A, B).
The RF power was progressively increased over 5-10 seconds to achieve a power of 3 W and then maintained for 180 seconds or until the current flow spontaneously collapsed. A lesion was considered complete when an abrupt rise in impedance and fall in current was observed, indicating that the coagulation necrosis was complete. Usually, the patient reported an audible crackling sound when the current flow suddenly stopped. This protocol was adopted based on in vitro and in vivo observations of Staudt and Miller, with the largest confluent lesions between adjacent electrodes created using a power of 3 W at all inter-electrode distances up to 12 mm, compared to higher-power settings [13]. Staudt and Miller also observed that using higher RF power actually produced smaller lesions, so power was maintained below 3 W for all lesions produced in this study.
Clinical assessment after SSEG-guided radiofrequency transection
Once the lesions were created, the patients remained in the epilepsy monitoring unit for an additional three to five days of recording. After the ablation, the antiepileptic medication was continued at lower doses than home doses for the remaining days of the monitoring to assess for recurrence of spontaneous seizures. Incomplete perpendicular disconnection within the amygdalo-hippocampal complex was suspected if after ablations, seizures were recorded. Therefore, additional SEEG-guided-RF ablations were performed in an attempt to achieve complete disconnections. Each patient was discharged on either full home dose of antiepileptic medication or a reduced dose if supported by results of additional EEG monitoring. We evaluated post-procedure seizure outcome and the safety of the procedure.
Results
Complications
All patients tolerated the procedure well and there were no complications related either to the implantation of the depth electrodes or the SEEG-RF-MHT.
Seizure outcome
Two ablation sessions were performed in each case because of continued electrographic or clinical seizures during the first 24-48 hours following the first ablation. Long-term seizure outcome is summarized in table 2 table 2. Three out of five patients were seizure-free with a follow-up of 17-24 months. One additional patient was seizure-free but was operated on in June, 2020.
Subject 4 underwent bilateral temporal lobe implantation and indeed had seizures arising from both temporal lobes when the antiepileptic medication was completely discontinued. However, on antiepileptic medication, the patient had only seizures arising from the left temporal lobe. Since the seizures arising from the left hippocampus were occurring daily and the seizure from the right-sided focus were controlled on antiepileptic medication, SEEG-guided RF-MHT was performed on the dominant (left) amygdalo-hypocampal complex. This patient is currently 17 months seizure-free and benefited from the procedure since he had been suffering from daily debilitating seizures (one automotor seizure with dialepsis per day). Since this procedure was offered to decrease seizure frequency in his particular case, the patient was informed of the need to remain on life-long antiepileptic medication to control the epileptogenic focus on the right side.
Subject 2 reported having auras and new-onset episodes at three months after the procedure. The patient was monitored on three different occasions. Antiepileptic drugs were weaned off during the three evaluations and no evidence of epileptogenicity was found. Indeed, multiple episodes without EEG correlate were recorded and, therefore, this subject was diagnosed with paroxysmal non-epileptic events and was considered seizure-free.
Subject 1 was the only patient in our series of five cases who had recurrence of seizures four months after the procedure. Initially, it was thought that the seizures were associated with alcohol intake. However, later on, the patient reported seizures occurring spontaneously. A surveillance EEG, obtained six months after the procedure, revealed continuous periodic activity (without clinical manifestations) in the left anterior-temporal region suggesting that most likely the disconnection of the amygdalo-hipoccampal complex was incomplete. It is important to note, however, that in this case several critical electrode contacts were damaged at the time of the SEEG-guided-RF-MHT. Consequently, a complete transection was not achievable.
We were able to reduce the dose or number of drugs prescribed in two patients during follow-up appointments (at six months post SEEG-guided-RF-MHT procedure in Subject 3 and at one year in Subject 2). These two patients remain seizure-free at 18 months and 24 months of follow-up, respectively. Subject 5 was discharged on a reduced number of antiepileptics since a significant decrease in interictal activity was observed after the procedure. This patient has been seizure-free for six months after the SEEG-guided-RF-MHT.
Neuropsychology
Postoperative neuropsychology data will be compared with preoperative baselines and will be reported in a separate paper. Subjects who received the procedure in the dominant lobe (Subjects 1, 3, 4 and 5) reported no subjective memory complaints in the follow-up clinic assessments at six months (Subject 3) and one year (Subject 1 and 4). During a follow-up telephone call at three months post-procedure, Subject 5 reported no subjective memory complaints. When these patients were asked to judge their memory, they reported no change before and after the procedure. Patients denied any problems remembering names, faces, conversations, appointments, personal dates and words or with regards to finding things and direction to places. This was also confirmed by relatives.
Post-operative brain MRI
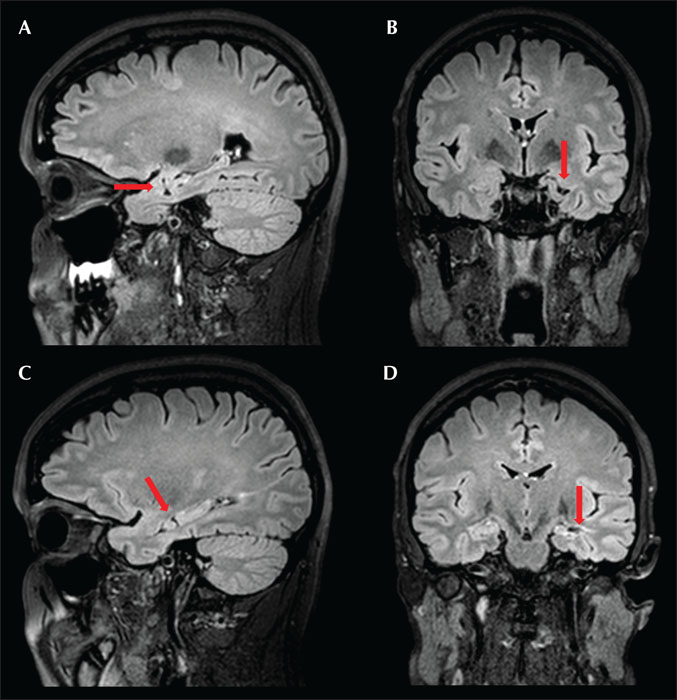
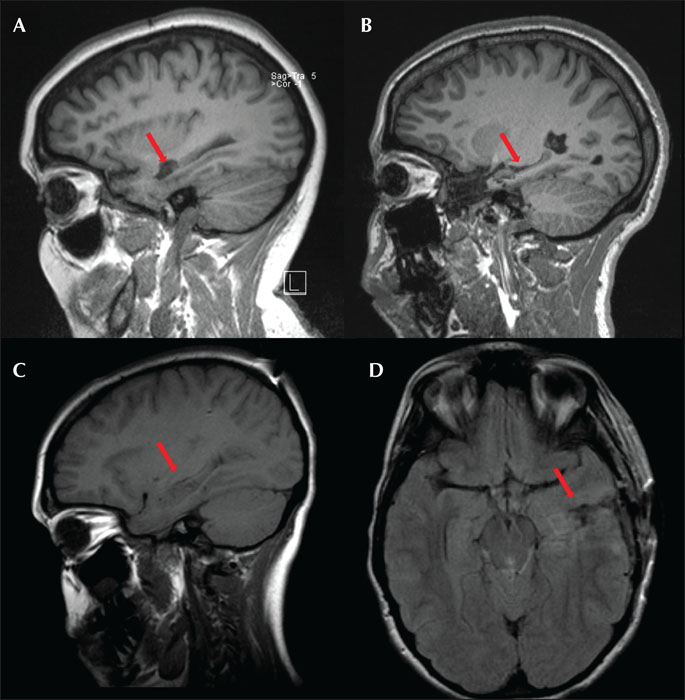
Visual inspection of brain MRI of patients at six months following SEEG-guided RF-MHT showed significant hippocampal volume preservation (figure 2), similar to that seen after standard MHT alone with no resection of brain tissue (MHT-). The advantage of SEEG-guided-RF-MHT is that this technique avoids iatrogenic neocortical injury that can be associated with the conventional approach via corticotomy in the middle temporal gyrus or a modified approach via resection of the temporal tip neocortex and the amygdala (MHT +) (figure 3).
Discussion
We describe a new strategy of SEEG electrode placement and coagulation that allows the production of two to three linear confluent RF ablations, perpendicular to the longitudinal axis of the hippocampus, to limit damage of the amygdala-hippocampal complex in order to minimize the risk of postoperative memory decline of patients with dominant mesial temporal lobe epilepsy with high memory scores. Our data on preliminary seizure outcome in this small cohort is encouraging, moreover, memory performance was reported to be unchanged by the patients.
The complications of standard temporal lobe resections may be multiple, including verbal memory decline and linguistic disturbances when surgery is performed on the language dominant side [17, 18]. In the past decades, several procedures have been developed with a goal to selectively target mesial structures (amygdala and hippocampus) with different approaches, aiming to minimize memory decline, especially in patients with preserved memory function [19]. Table 3 shows different types of surgical techniques targeting the amygdalo-hippocampal complex, post-surgical seizure outcome and the potential type of memory decline associated with each procedure in patients who received surgery on the language-dominant side. Of note, the results shown in table 3 are extracted from a review of the literature.
In this report, we present a treatment technique, SEEG–guided RF-MHT, targeting only the longitudinal fibers in the hippocampus that are most likely critical for seizure synchronization and spreading [21]. This procedure consists of two to three linear coagulative lesions perpendicular to the longitudinal axis of the hippocampus, sparing (as in the MHT classic technique) the transverse circuits and their inputs from the entorhinal cortex which are considered important for memory processing [22].
This technique has several advantages when compared to other established techniques for the treatment of epilepsy arising from language-dominant mesial temporal structures in patients with normal or high memory scores. The potential advantages of SEEG-RF-MHT are summarized below:
- •(1) The use of SEEG electrodes for performing thermo-coagulative lesions requires only a one-step procedure for both diagnosis and treatment. SEEG-guided-RF-MHT does not require craniotomy, thus shortening the postoperative recovery time and avoiding the possible complications related to craniotomy.
- •(2) Our preliminary seizure outcome seems very promising since the majority of our patients (four out of five patients) were seizure-free at 6, 17, 18 and 24 months of follow-up, respectively.
- •(3) The electrophysiological status of the patient can be monitored in real-time before, during, and after the lesion. Video-EEG monitoring can be performed for several days after the SEEG-RF-MHTs are performed. Additional SEEG-guided-RF lesions can be made during the same admission if the video-EEG monitoring reveals persisting epileptiform discharges, SEEG seizures, or even clinical seizures. The location of the persisting epileptiform activity can be used as a guide to determine the location of the additional RF lesions.
- •(4) The treatment with relatively limited thermo-lesions does not preclude subsequent conventional surgery in case of failure.
As mentioned above,table 3 summarizes the different surgical procedures that have been applied in an attempt to control seizures in patients with mesial temporal lobe epilepsy, while minimizing post-surgery memory and language deficits. Potential advantages using the SEEG-RF-MHT technique with regards to neurocognitive outcome are as follows:
- •(1) Three of the surgical techniques developed to treat MTLE in patients with high memory scores (anterior temporal lobectomy [ATL], SAH and stereotactic laser amygdalo-hippocampotomy [SLAH]) destroy the amygdalo-hippocampal complex completely or almost completely and the expected degree of verbal memory decline in these patients is greater than that expected with MHT or SEEG-guided-RF-MHT [17-91, 23, 24]. The cognitive outcomes of LiTT ablation for higher-risk patients remains unclear. Jermakovicz et al. found that 20% of patients with dominant-hemisphere ablation declined based on verbal memory test after SLAH. In this cohort of patients, 90% of the language-dominant patients had pre-surgical memory scores in the lowest quartile compared to normative samples [25].
- •(2) The hippocampus contains two types of circuit: (i) transverse lamellae arranged in parallel loops that originate and end in the entorhinal cortex (their pathways run across the longitudinal axis of the hippocampus) and are important for memory processing [22], and (ii) longitudinal pathways that have been shown to be important for synchronization of seizure discharges [21]. MHT is a tailored procedure in which six to seven complete transverse transections are performed in the hippocampus with the aim of preserving functional tissue in order to minimize damage to the hippocampal-amygdaloid complex. However, MRI analysis of a cohort of eight patients showed that the ratio of hippocampal volume on the operative to non-operative side decreased significantly after hippocampal transections [4]. The decrease in hippocampal volume can be seen in the post-operative brain MRI of our patients who underwent MHT (+) via craniotomy in our center (figure 3). Visual inspection of the MRI of patients who had MHT(+) and those who had SEEG-guided-RF-MHT (figure 2) indicates that, in general, SEEG-guided-RF-MHT produced significantly less damage to the hippocampus. Despite this significant decrease in hippocampal volume seen in our cohort of patients who had MHT (total of 26 patients), 72 % remain seizure-free after two years and verbal memory decline was found in 23% of the patients at high risk of verbal memory decline vs the average 45% found in temporal lobectomies [26]. On the other hand, 47% of patients had language decline noted on the Boston naming test, which approximates the ATL average but which is lower than the 75% expected based on specific risk factors in this cohort (Fastenau et al. [abstract] in press, Epilepsia). It is possible that the sparing of the entorhinal cortex with this technique led to better neurocognitive outcome. This would support the hypothesis of Andersen et al. in 1969 who postulated the “lamellar hypothesis” of hippocampal function in which interactions between the entorhinal cortex and dentate gyrus, via perforant path fibers, are organized topographically, and that “lamellae” (a series of parallel hippocampal “slices”) might operate independently. It is also possible that language decline found in this cohort of patients, who underwent MHT via craniotomy, was related to the extent of resection of the temporal neocortex, and that in some patients, this was associated with MHT (+) or the approach through the temporal stem itself.
- •(3) All procedures (ATL, SAH and MHT) except SLAH and SEEG-guided-RF-MHT produce some degree of damage to the temporal neocortex. Sparing of the temporal stem white matter, the parahippocampal gyrus and the lateral gyri (e.g. fusiform gyrus) could be related to the good outcome for category-related recognition (contextual memory) and naming abilities in patients who undergo SLAH vs those receiving standard resections (tailored or open ATL or SAH) [27]. In five patients who underwent SLAH on the dominant side, there was no change on the Weschsler Memory Scale (WMS-IV) logical memory (LM) I and II test, but three of the five (60%) declined significantly based on another verbal memory test (CVLT-II) [28]. The authors hypothesized that contextual memory tested using the WMS LM task was less affected by SLAH due to preservation of lateral temporal language processes, suggesting that semantic aids may play a role in memory acquisition for this task. In contrast, non-contextual processes tested using the CVLT (California Verbal Learning Test), not aided by semantic context, reveal the effects of SLAH directly in the hippocampus more effectively. In the same way, it seems reasonable that patients undergoing SEEG-guided RF-MHT may have better neurocognitive scores in both contextual and non-contextual learning processes and language abilities, since this technique preserves the temporal stem and amygdalo-hyppocampal functions. Moreover, it is reasonable to think that neurocognitive outcomes will be at least as good as in patients who have undergone MHT since SEEG-guided RF-MHT causes less damage to the hippocampus and entorhinal area. Transient verbal memory decline following implantation of depth electrodes to the longitudinal axis of the hippocampus has been described elsewhere, however, patients seemed to recover from the implantation effect [29].
In summary, there are multiple advantages of SEEG-guided-RF-MHT for the treatment of MTLE. The presented method can be considered as more of a surgical technique than a diagnostic tool in patients with clear mesial temporal lobe epilepsy based on the pre-surgical work-up, and usually patients with all diagnostic data merging to the mesial temporal lobe are treated surgically without any prior invasive investigations. In patients in which the pre-surgical data are not completely supportive of MTLE (as in our patients with high memory scores and almost normal MRI), but the epileptogenic zone is still considered within the temporal lobe, the placement of SEEG electrodes only within the temporal lobe is justified for defining the epileptogenic zone and for treatment options. Post-ablative continuous SEEG data helps to evaluate the effect of the transablations. This procedure only targets and disconnects longitudinal fibers in the hippocampus likely responsible for seizure spread without disruption of transverse memory circuits and their connection to the fimbria and entorhinal area. Moreover, no lesion is performed outside the amygdalo-hippocampal complex, and the temporal stem remains intact. These much-targeted lesions may produce a better memory and language outcome in patients who have high risk of memory decline after hippocampal resection or complete coagulation. The small number of patients included in this study and the short follow-up period makes it difficult to extract statistically significant differences between this new procedure and the traditional treatments.
Supplementary material
Summary slides accompanying the manuscript are available at www.epilepticdisorders.com.
Disclosures
None of the authors have any conflicts of interest to disclose.