Epileptic Disorders
MENUPanic attack semiology in right temporal lobe epilepsy Volume 5, numéro 2, June 2003
Auteur(s) : Mona Sazgar, Peter L. Carlen, Richard Wennberg
Krembil Neuroscience Centre, Toronto Western Hospital, University of Toronto, Toronto, ON, Canada
Correspondence: Richard Wennberg, MD, FRCPC Division of Neurology, Toronto Western Hospital, 399 Bathurst Street, 5W444, Toronto, ON, Canada M5T 2S8 Tel.: (416) 603-5402 Fax: (416) 603-5768 E-mail: r.wennbergutoronto.caPresented in part at the American Epilepsy Society meeting, December 2001, Philadelphia, PA, USA
Received December 27, 2002; Accepted April 17, 2003
Introduction
Some symptoms of panic attacks such as anxiety, fear, derealization or depersonalization, altered autonomic functions and cardiovascular fluctuations may be found both ictally and interictally in epileptic patients. Several animal and human studies have provided evidence for right hemispheric dominance in the cerebral control of heart rate and blood pressure modulation, typically ascribed to sympathetic lateralization in the right hemisphere [1-5], although parasympathetic effects have been implicated by others [6]. Although it is recognized that some symptoms of complex partial seizures overlap with panic and autonomic symptoms, there are relatively few documented cases of panic disorder that are later found to be due to focal epileptic disorders. We present a case series to demonstrate the association of ictal panic and anxiety symptoms with partial onset seizures lateralized to the right temporal lobe. We review the relevant literature.
Methods
From 112 consecutive patients with intractable temporal lobe epilepsy (59 right, 53 left) referred for video-EEG monitoring at the Toronto Western Hospital between February 1997 and April 2001, five patients were identified whose seizures had been diagnosed as panic attacks at some point in the past. A history of ictal panic was not identified in 72 patients with extratemporal epilepsy investigated during the same period.
Results
All five patients were ultimately diagnosed with right temporal lobe epilepsy. The individual case histories are given below. The apparent trend to a right temporal localization and lateralization for ictal panic in this study group did not reach statistical significance (0.05 < P < 0.1, Chi-Square test, two-tailed, with correction for continuity).
Patient 1
Patient 1 was a 48-year-old man whose first seizures had
occurred at age 16. Investigations led to the discovery of a right
temporal lobe tumor, which was resected. Pathology was consistent
with ganglioglioma. Following the resection of his tumor, over the
next 20 years, he experienced paroxysmal, brief (15 –
30 s) episodes of sudden onset palpitations, diaphoresis,
shortness of breath, generalized paresthesias, anxiety and feelings
of warmth. These episodes occurred two to three times a week. He
was treated with phenytoin for many years, and subsequently
switched to carbamazepine.
Ten years prior to investigations at this hospital, he began to
experience lapses of awareness during some of his spells and he
started to have more frequent attacks, which could not be
completely controlled with valproate, lamotrigine, clobazam,
gabapentin, vigabatrin, or various combinations thereof. He was
also followed for chronic depression and anxiety. Medically-trained
witnesses of some of his attacks had documented their uncertainty
as to whether they had witnessed simple partial seizures or panic
attacks.
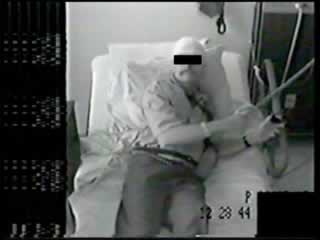
At the time of admission to the Epilepsy Monitoring Unit (EMU) for
video-EEG investigation, he was experiencing 3 – 6 events
per day. Sixteen partial onset seizures were recorded during his
EMU admission. During these episodes he activated the event marker
as he sensed his aura of fear, and subsequently had an anxious
expression on his face. He then experienced deep regular breathing
and was able to verbalize that he felt anxious, that he was
hyperventilating, and that his heart was pounding. With larger
events, he stated that he was unable to think clearly and would
stop what he was doing until the end of the spell. Ictal, or, more
frequently, postictal noserubbing [7] with the right hand
accompanied some of the events (see videosequences).
In all seizures, the first definite ictal electrographic activity
was preceded by the onset of clinical symptomatology. Seizure
onsets were localized to the right midtemporal region as low
amplitude, 10 – 14 Hz rhythmic ictal activity with phase
reversal at T4 (figure
1). Brain MRI showed the previous right anterolateral
temporal resection with sparing of the mesial temporal structures
(figure 2). The
patient underwent repeat right temporal lobe surgery, this time
with amygdalohippocampectomy and further resection of the
structures posterior to the previous excision (figure 3).
Electrocorticography prior to this resection revealed active
spiking from the second and third temporal gyri at the posterior
margins of the previous resection and overlying the preserved
amygdala and anterior hippocampal structures. Surgical pathology
demonstrated traces of residual ganglioglioma in a parahippocampal
specimen from the posterior portion of the resection.
Following this second surgery, the patient remained seizure-free
on valproate monotherapy for nearly two years. Seizures
subsequently recurred, however, initially as infrequent nocturnal
convulsions over the next two years. During the fifth postoperative
year diurnal seizures returned, no longer associated with panic
symptomatology or aura of any kind. Reinvestigation with continuous
ambulatory EEG at this time captured two such seizures, both of
right temporal origin with subsequent spread to the left temporal
lobe, now associated with ictal bradycardia (figure 4).
Patient 2
Patient 2 was a 25-year-old woman, first diagnosed with
panic attacks at the age of 16. Her attacks consisted of brief
episodes of hyperventilation, palpitations, feelings of fear and
impending doom, lasting between 30 s to 2 min. Three
years later, a diagnosis of epilepsy was reached when oral
automatisms and staring were noted and treatment was started with
antiepileptic medications. She was allergic to phenytoin and
carbamazepine, and did not tolerate valproate, clobazam or
topiramate. When admitted to the EMU for localization of her
seizures, she was taking lamotrigine monotherapy with spells
occurring approximately five times per week.
There was also a recent history of episodes beginning with her
typical subjective symptomatology, but progressing to involve
unusual movements of all extremities, not associated with
alteration of consciousness according to the patient’s own
description, raising the possibility of additional nonepileptic
events. The onset of these episodes had been noted to coincide with
the patient’s leaving home to live with her boyfriend and the
concurrent commencement of a new job, both of which had been
significant emotional stressors.
During 6 days of video-EEG monitoring, three types of events
were recorded. The first episode was marked only by
hyperventilation, lasting approximately 2 min, with no EEG
changes. Subsequently, four episodes were recorded commencing with
hyperventilation followed by long periods, lasting more than
5 min, of repetitive bilateral finger flexion and extension
progressing to large amplitude athetoid movements of both arms and
legs, all in the setting of completely preserved awareness and
responsivity. There were no associated ictal EEG changes with these
events. Ultimately, two complex partial seizures were recorded,
associated with early hyperventilation (approximate doubling of the
baseline respiration rate from 18 to 32 per minute during
the first 60 s of the clinical seizure, preceeding the first
ictal electrographic changes by 30 s), tachycardia, oral
automatisms and eye deviation to the left. Two secondarily
generalized seizures were then recorded, both of which arose from
sleep. Ictal hyperventilation during sleep could not be appreciated
on video prior to the arousal associated with generalization in
these two seizures.
The ictal electrographic onsets were all localized to the right
anterior temporal region. The spells unassociated with EEG changes
were interpreted to represent nonepileptic events, although the
possibility of psychogenic elaboration of simple partial seizures
not apparent on scalp EEG, could not be excluded [8, 9]. MRI showed
right mesial temporal sclerosis. The patient underwent a right
selective amygdalohippocampectomy and has been free of all episodes
for more than 18 months.
Patient 3
Patient 3 was a 34-year-old woman with a normal birth
history and uneventful childhood. At age 20, she developed
persistent headaches and was diagnosed with hydrocephalus
attributed to congenital aqueductal stenosis. A right-sided
ventriculo-peritoneal shunt was inserted and revised four years
later. That same year, when pregnant, she experienced her first
seizure, a generalized convulsion. She was started on
phenobarbital, switched to phenytoin, and then to carbamazepine and
clobazam. During the year before her admission to the EMU, she was
experiencing episodic olfactory hallucinations of “medicinal”
smells described to last 1 to 2 min, followed by a
feeling of fear lasting 30 min with her whole body feeling
numb, and her head heavy. These events occurred 2 to
3 times a day. Over the same year, she had also experienced
depression and decreased emotional control. She was seeing a
psychiatrist for these problems and had been given a diagnosis of
major depression, anxiety disorder and panic attacks. The patient
was reluctant to take the prescribed psychotropic medications.
The patient was referred to the EMU for diagnostic purposes. After
discontinuing her antiepileptic drug therapy, a number of simple
partial seizures were recorded associated with her typical aura of
a medicinal smell and a feeling of fear, all localized to the right
anterior temporal region. No complex partial seizures were
recorded. MRI showed right mesial temporal sclerosis, as well as
encephalomalacia along the right occipital ventricular shunt
insertion. She is currently taking carbamazepine monotherapy, with
infrequent seizures, and does not want to consider surgical
management at this time.
Patient 4
Patient 4 was a 39-year-old woman, first diagnosed with panic
attacks at age 9. Her episodes were characterized by feelings of
intense fear, panic and palpitations, lasting about 2 min.
Years after the onset of her spells, automatisms and occasional
falls were noted and she was diagnosed with epilepsy. Her events
were significantly more frequent near the beginning of her menses.
She had a long history of diagnosed anxiety treated with
anxiolytics, with what amounted to agoraphobic behavior
perimenstrually, her panic and anxiety at these times raising the
suspicion, in retrospect, of an epileptic “aura continua” state.
Treatment with various antiepileptic drugs invariably decreased the
frequency of events but never completely eliminated them, and she
tolerated the medications poorly.
Video-EEG monitoring documented right temporal lobe ictal onsets
for her typical events, with an exceedingly active unilateral right
anterior temporal interictal epileptiform abnormality, at times
recorded continuously for minutes on end, giving credence to the
possibility of an “aura continua” etiology to her periods of
agoraphobic behavior (figure 5). MRI showed right
mesial temporal sclerosis. Initially reluctant to consider surgical
management, she is now awaiting right anterior temporal
resection.
Patient 5
Patient 5 was a 24-year-old woman with a history of two febrile seizures during infancy. She was treated with phenobarbital until age 8, though there was no seizure recurrence until age 20. Typical events consisted of an aura of a “head rush” quickly followed by progression to complex partial seizures associated with hypersalivation and terminating with a cough. One prolonged event, which had commenced with the same aura, did not progress to the typical complex partial seizure but instead was followed by pronounced hyperventilation and fear as well as an altered sense of visual perception suggestive of depersonalization or derealization. This event led to her being taken to a hospital emergency department for evaluation, where a diagnosis of panic attack was offered.
The patient was referred for video-EEG monitoring after trials of multiple, different antiepileptic drugs (valproate, gabapentin, topiramate, carbamazepine, and combinations thereof) had not been able to completely control her seizures. Three of her typical complex partial seizures were recorded with a brief aura prior to loss of awareness with blank stare, postictal noserubbing and postictal coughing [10]. Ictal onsets were lateralized to the right frontotemporal area, maximal over the anterior temporal region. MRI showed right mesial temporal sclerosis. The patient subsequently underwent a right selective amygdalohippocampectomy and has been seizure free more than 18 months after her operation.
Discussion
Partial onset seizures presenting with panic attack
symptomatology may pose a diagnostic challenge. Fear, an affective
symptom associated with idiopathic panic attacks, is also seen as
an ictal symptom in 10 15% of patients with temporal lobe
epilepsy [11]. Fear was described to be the most common
experiential phenomenon produced by direct electrical brain
stimulation in temporal lobe epilepsy, localized to the
anteromedial temporal region including the amygdala [12]. Other
than fear, focal seizures and panic attacks may both present with
autonomic symptoms such as tachycardia, blood pressure
fluctuations, hyperventilation and dyspnea. Our case series
demonstrates this overlap of symptomatology and the need to
differentiate between these two distinct diagnostic entities, which
have very different treatment implications.
Hemispheric control of autonomic function has been the subject of
debate for many years. Despite some contradictory results in the
literature [6], several investigators have shown right hemispheric
dominance for sympathetic heart rate and blood pressure modulation.
Hilz et al. analyzed autonomic heart rate and blood pressure
modulation in 15 patients with refractory epilepsy during
hemispheric inactivation with the intracarotid amobarbital
procedure (IAP) [1]. They confirmed previous IAP studies indicating
sympathetic lateralization in the right hemisphere and
parasympathetic predominance in the left hemisphere [4, 5]. In
patients with right hemispheric partial onset seizures, it is then
conceivable that ictal electrical stimulation of ipsilateral
hemispheric autonomic centers could evoke tachycardia,
hyperventilation and other symptoms resembling panic semiology. The
principal site of autonomic representation in the brain is thought
to involve the insular cortex [1-3]. Spread of right mesial
temporal ictal activity to insular cortex may be responsible for
the autonomic panic attack symptomatology, although this remains
speculative and other possible networks of activation (for example,
direct temporolimbic-hypothalamic pathways) may be responsible.
Our demonstration in patient 1 that panic symptomatology and
ictal tachycardia both disappeared after right mesial temporal
resection of uncus/amygdala, anterior hippocampus and adjacent
parahippocampal gyrus strongly supports the preoperative
localization of initiation of these symptoms and signs within the
structures subsequently resected. Eventual recurrence of seizures
originating from the residual posterior temporal lobe structures on
the right side, now associated with ictal bradycardia during
seizure spread to the left temporal region, suggests that only the
left hemispheric (parasympathetic) autonomic centers could now be
activated by the ictal activity: ie. that the right anteromesial
temporal region was necessary for ictal activation of the right
hemispheric autonomic centers
Changes in respiratory rate were not found with inactivation of either hemisphere in one study using the IAP [1]. This raises the possibility that ictal hyperventilation may not necessarily represent direct ictal effects upon autonomic centers, but may instead arise in response to the affective feelings of fear and panic. The observation that hyperventilation was not seen in patient 2 with seizures starting in sleep, but was clearly evident with diurnal events, lends some support to the possibility of an affective component to ictal hyperventilation.
Diagnostic difficulties are not limited to the clinical differentiation of attacks. Simple partial seizures unassociated with EEG changes may be misinterpreted as nonepileptic events if their symptomatology is entirely compatible with panic attacks [13]. Likewise the scenario of psychogenic elaboration of simple partial seizures unassociated with EEG changes [8, 9]. Patient 2, in all likelihood, demonstrated both of these phenomena prior to the video-EEG confirmation of an epileptic etiology obtained with the recording of larger events. Although it is presumably only a small proportion of patients diagnosed with anxiety and panic attacks who are actually suffering from a focal epileptic disorder, the possibility of an epileptic “aura continua” etiology for even prolonged periods of pathological anxiety is suggested by the “interictal” EEG findings in patient 4.
There are previous reports in the literature describing patients with lateralized epilepsy initially presenting as panic disorder [13-26]. It has been suggested that partial seizures initially misdiagnosed as panic attacks may be a clue to the diagnosis of symptomatic right temporal lobe epilepsy [19]. Although all five of the patients reported in this study did suffer from right temporal lobe epilepsy, this lateralizing trend did not reach significance. However, a review of the previously published cases of definite epilepsy (those with well-described scalp or intracranial EEG plus or minus concordant neuroimaging findings [13-21]) does show that in a vast majority of patients, a right hemispheric, and especially temporal lobe, focus for the epileptic disorder was identified, with 83% of the reported patients found to have a structural lesion (table 1). Combining the results from these studies does show a significant bias for a right hemispheric lateralization (20:3, right:left; P < 0.001, Chi-Square test, two tailed, with correction for continuity). Notwithstanding, review of the additional published cases of possible or questionable epilepsy presenting as panic attacks [22-26] does not show evidence of lateralization (table 2), and the existence of proven cases with extratemporal or left temporal localization [16, 18, 20, 21] shows that the association of panic attack semiology with right temporal lobe epilepsy is not invariant. Seizure spread, to preferentially activate right hemispheric autonomic centers, might underlie panic symptomatology in patients with extratemporal or left temporal onsets. However, it is equally possible that individual differences in lateralization or localization of autonomic centers could be responsible.
Table 1. Reported cases of probable and definite epilepsy presenting with panic semiology.
| Reports | Patients | R Temporal | Other Sites | Structural lesions |
|---|---|---|---|---|
| Ghadirian et al. (1986) | 1 | 1 | 0 | Meningioma |
| Devinsky et al. (1989) | 1 | 1 | 0 | None |
| Laidlaw et al. (1993) | 1 | 1 | 0 | None |
| Alemayehu et al. (1995) | 2 | 0 | 2 R parietal | Astrocytoma |
| Meyer et al. (2000) | 1 | 1 | 0 | MTS |
| Daly et al. (2000) | 1 | 0 | 1 R frontal | Cortical dysplasia |
| Tassinari et al. (2000) | 7 | 7 | 0 | 2 MTS, 2 cavernomas, 2 tumors, 1 cerebral lupus |
| Thompson et al. (2000) | 3 | 1 | 2 L temporal | 1L MTS, 2 None |
| Huppertz et al. (2002) | 1 | 0 | 1 L temporal | Cortical dysplasia |
| Sazgar et al. (2003) | 5 | 5 | 0 | 4 MTS, 1 ganglioglioma |
| Total | 23 | 17 | 6 | 19 |
Table 2. Reported cases of possible/questionable epilepsy associated with panic attacks.
| Reports | Patients | R Temporal | Other lateralized sites | Bilateral seizure onsets |
|---|---|---|---|---|
| Wall et al. (1985) | 1 | 1 | 0 | 0 |
| Weilburg et al. (1987) | 3 | 1 | L temporal; 1 L hemisphere | 0 |
| Edlund et al. (1987) | 6 | 3 | 2 L temporal | 1 bifrontotemporal |
| Dantendorfer et al. (1995) | 3 | 1 | 1 L parieto-occipital | 1 bitemporal |
| Pegna et al. (1999) | 1 | 0 | 1 L temporal | 0 |
| Total | 14 | 6 | 6 | 2 |
In keeping with an asymmetry of hemispheric representation for panic symptomatology Reiman et al., using positron emission tomography to measure baseline cerebral blood flow in patients with idiopathic panic disorder, demonstrated increased blood flow in the region of the right parahippocampal gyrus [27]. Panic symptomatology in both epileptic and nonepileptic patients may thus implicate structural or functional alterations similarly localized to the right mesial temporal region. Activation of the same temporolimbic structures could contribute, via different mechanisms, to the pathophysiology of both idiopathic panic disorder and epileptic panic symptomatology.
In our case series, the clinical history provided important clues to the diagnosis of epilepsy such that by the time of our assessment diagnostic uncertainty was not an issue. In the early stages of presentation, however, diagnostic uncertainty in similar patients is to be expected. The past medical or surgical history contributed to the diagnosis in three of our five patients and included febrile convulsions, previously resected brain tumor, and shunted hydrocephalus (though the latter condition is presumably unrelated to the subsequently discovered mesial temporal lobe epilepsy and sclerosis). Other differentiating factors in favor of a diagnosis of epilepsy as opposed to idiopathic panic attacks, include short duration of attacks, stereotyped aura (apart from fear), ictal unresponsiveness, oral or motor automatisms, postictal confusion, nocturnal occurrence, lack of response to anxiolytic medications and, most importantly, EEG confirmation of the diagnosis of epilepsy. o
 Video
Caption Video
Caption |
|---|
| • First seizure: Clinical
onset approximately 12:28:39 • Scalp EEG onset 12:28:45 • (No speech) • Second seizure: Clinical onset approximately 15:48:05 • Scalp EEG onset 15:48:15 • 15:48:10 Hangs up phone • 15:48:54 “Bad one...” • 15:49:03 “More and more anxious...” • 15:49:07 “My heart’s picked up...” • 15:49:15 “...can’t think clearly...” • 15:49:28 “...dizzy...funny with vision...” • 15:49:43 Phone rings “Hello...Yeah...I’ll call you back. Bye” Hangs up phone • 15:49:53 “Starting to tremor now...hotter...” • 15:50:05 “There’s some relief...the panic is going...” • 15:50:24 “I hung up on the phone...wasn’t thinking clearly” • 15:50:53 “It’s gone now” • Third seizure: Clinical onset approximately 19:29:21 • Scalp EEG onset 19:29:26 • 19:30:19 “Heart is pounding...” • 19:30:36 “Boy oh boy...” |