Epileptic Disorders
MENUMapping of cingulate motor function by cortical stimulation Volume 15, numéro 3, September 2013
epd.2013.0595
Auteur(s) : Maysaa M Basha1 mmerhi@med.wayne.edu, Guadalupe Fernández-Baca Vaca2, Hans O Lüders2
1 Department of Neurology, Wayne State University, Detroit
2 Department of Neurology, Case Western Reserve University, Cleveland, USA
Correspondence. Maysaa M Basha Department of Neurology, Wayne State University, University Health Center, 8C, 4201 Saint Antoine, Detroit, MI 48201, USA
Direct electrical stimulation and mapping of the cerebral cortex in both humans and the non-human primate has helped to establish the cortical representation of motor function, located to two main areas of the cerebral cortex: the primary motor cortex and the supplementary sensorimotor area (SSMA). A third motor representation has been identified along the banks of the cingulate sulcus in the non-human primate (Dum and Strick, 1991; Luppino et al., 1991; Matelli et al., 1991). In humans, cingulate motor representation has been demonstrated in epilepsy patients undergoing extraoperative electrical stimulation prior to resective surgery. (Diehl et al., 2000; Chassagnon et al., 2008). We report a case of cortical stimulation and mapping of the cingulate gyrus, revealing detailed and precise topographic motor representation, localised by co-registration of pre-insertion MRI and post-electrode implantation CAT in a patient undergoing epilepsy surgery.
Case study
An 8-year-old, right-handed boy presented with non-lesional intractable epilepsy. Seizures were characterised as brief bilateral asymmetric tonic stiffening, involving all four extremities in different combination and lasted for 5 to 30 seconds without any alteration of consciousness since the age of 2. The patient had normal development showing no risk factors for epilepsy. Neurological examination was normal. Neuroimaging including 3 Tesla MRI and FDG PET were normal. During surface video-EEG monitoring, spikes, maximal at Cz and FCz, were observed and the typical clinical seizures (∼30 seizures per day) were recorded with maximum electrographic discharge at Cz and FCz. He subsequently underwent invasive monitoring whereby subdural electrodes were placed on the mesial and lateral convexity of both frontal lobes, and evaluation revealed left mesiofrontal epilepsy with ictal onset anterior to the pre-supplementary sensorimotor area on the left. Subdural electrodes overlying the right mesial and lateral convexity of the frontal lobe were not part of the ictal onset zone. The patient had a small resection within the left mesial frontal lobe in the prefrontal cortex, immediately anterior to the pre-SSMA and superior to the cingulate gyrus. He did not present with any postoperative neurological deficit, but continued to have seizures which were typical of preoperative semiology.
One year later, he underwent re-evaluation for epilepsy surgery. Evaluation in the epilepsy monitoring unit revealed unchanged seizure semiology with interictal epileptiform discharges and the appearance of a slightly more anterior seizure onset zone, with a distribution in F3 and FC3. Focal slowing-down in the left frontocentral region was also observed, consistent with the patient's craniotomy and resective surgery. MRI revealed a resection cavity measuring 2 cm×2.5 cm in the sagittal plane and 2 cm×2 cm in the coronal plane within the prefrontal cortex, anterior to the pre-SSMA and superior to the cingulate gyrus. The patient underwent two-step presurgical evaluation using intracerebral depth electrodes, and further resection and pathology yielded a Palmini type II cortico-dysplasia. Postoperatively, the patient had recurrent seizures with decreased frequency.
The patient underwent further invasive monitoring with intracerebral depth electrodes placed around the resection cavity to better localise the epileptogenic zone. Four depth electrodes, arbitrarily referred to as AD, BD, DD, and ED, each with 8 to 10 contacts (AD1-8, BD1-8, DD1-10, and ED1-8), were stereotactically implanted with intraoperative electrocorticography and frame-based stereotaxy (figure 1). The implanted electrodes were made of platinum-iridium and each electrode contact was 2.5 mm in length, with 5-mm centre-to-centre inter-contact distance. The ictal onset zone was localised to ED1 electrode within the cingulate gyrus and inferior to the SSMA proper on the left with no other electrode involvement, and the patient subsequently underwent wide peri-lesional and cingulate resection.
Prior to surgery, the patient underwent intracerebral EEG recordings and stimulation to aid surgical planning for resection of the epileptogenic zone, in line with previous descriptions (Schüle et al., 2008). Extraoperative mapping and cortical stimulation was performed using an Ojemann cortical stimulator (Integra Radionics, Inc) in order to deliver a biphasic square waveform at a frequency of 50 Hz, with 0.2-msecond pulse duration in 2-15-second trains and amperage ranging between 2 and 12 mA at increments of 1 mA. Electrical activity in the stimulated and surrounding electrodes was monitored for afterdischarges. BD7 was used as a reference after it was established that stimulation up to 20 mA produced no clinical signs or afterdischarges.
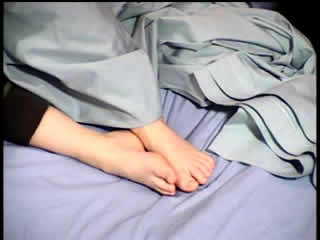
Stimulation of ED2 electrode contact with 9 mA resulted in mainly tonic flexion at the right elbow and wrist (video sequence 1). Stimulation of ED3 electrode contact with 11 mA resulted in mainly right foot dorsiflexion, right toe extension, with bilateral lower limb adduction (video sequence 2). These results were confirmed on two occasions, with a current intensity which was one to two-fold higher, with no afterdischarges noted and no change in clinical features. Stimulation of all other electrodes revealed no additional clinical features (figure 1). Image fusion software iPlan RT Image (BrainLAB AG) was used for co-registration of MRI obtained prior to insertion of depth electrodes with post-insertion CAT. Electrode contacts ED2 and ED3 were precisely localised to the ventral and dorsal banks of the cingulate sulcus, respectively (figure 2).
Discussion
Epilepsy patients have historically provided neurologists with an insight into the functional organisation of the cerebral cortex. Motor function was first attributed to the cerebral cortex via observational studies of focal progressive motor deficit by Paul Broca and motor seizure propagation by John Hughlings Jackson in the mid 19th century (Graziano, 2009). Almost a century later, Penfield and colleagues studied symptoms arising from seizures and, using applied direct cortical stimulation, were able to confirm the precise topography of motor function; the cortical representation was illustrated pictorially through the introduction of the human homunculus (Penfield and Rasmussen, 1950; Penfield and Jasper, 1954). The supplementary motor area was defined as a separate representation of motor function located on the mesial surface of the frontal lobe, anterior to the primary motor area (Penfield and Welch, 1951). Our case of direct cortical stimulation in an epilepsy patient demonstrates a cingulate motor area, somatotopically distributed along the banks of the cingulate sulcus, as seen in the non-human primate.
In the non-human primate, motor representation has been identified along the banks of the cingulate sulcus and defined based on histological, physiological, and functional studies. Histology of cytoarchitecture has shown that the cingulate cortex contains a distinct area, labelled as “area 24d”, with features consistent with motor function (Matelli et al., 1991). These features consist of increased size of pyramidal cells in layer V, an increase in the number of large pyramidal cells in layer III, and more pronounced radial vertical organisation of the deep layers. In addition, retrograde transport tracer experiments have demonstrated direct projections of the cingulate motor areas to both the primary cortex and spinal cord. Double-tracer injection of lower cervical segments and lower lumbar segments has revealed that the arm and leg representation in the cingulate motor areas is as spatially separate as that in the primary motor cortex (Dum and Strick, 1991). Furthermore, direct intracortical stimulation of the dorsal and ventral banks of the cingulate sulcus produced clonic movements that were less contiguous and less complex when compared to stimulation of SSMA proper in the same primates, and were somatotopically distributed (Luppino et al., 1991). Lower extremity representation was found to be mostly located in the upper (dorsal) bank and fundus of the cingulate sulcus and upper extremity representation, in parallel, in the lower (ventral) bank of the cingulate sulcus.
In humans, cortical stimulation of the anterior cingulate gyrus has produced complex behavioural manifestations (Bancaud et al., 1976), as well as an urge to grasp in a separate case (Kremer et al., 2001). More discrete SSMA-type motor responses have been produced in other experiments (Lim et al., 1994), however, imaging co-registration methods used by the authors did not allow for differentiation between stimulation of the inferior part of the mesial frontal gyrus and the cingulate gyrus. Another stimulation study of the cingulate gyrus using subdural electrodes in a single patient with epilepsy and a right frontal lobe mass revealed distal motor responses of the leg and arm upon stimulation of adjacent electrode contacts (Diehl et al., 2000).
A study investigating 52 patients, who underwent stereotactic electroencephalography recordings with depth electrode contacts that transversed the cingulate motor areas (CMA), SSMA proper, or pre-SSMA, confirmed the presence of cingulate motor function (Chassagnon et al., 2008). The CMAs were defined in relation to the cingulate sulcus and according to previous cytoarchitectural, neurophysiological, and functional studies and were then traced to Talairach anatomical landmarks. Twenty-four contacts overlying the CMAs produced a clinical response; 13 in the anterior CMA and 11 in the posterior CMA. Of these, tonic posturing of the limbs was produced on stimulation of 1 contact in the ventral bank and 1 contact in the dorsal bank of the posterior CMA, and 2 contacts in the dorsal bank of the anterior CMA. Additional tonic posturing was produced in combination with speech arrest in 2 other contacts which transversed the CMAs. Other clinical results elicited from stimulation of CMAs included limbs held in sustained position (behavioural arrest), eye and head deviation, speech arrest, reaching and grasping, an urge to laugh, and sensory symptoms.
The motor responses in our case mostly involved the distal segments of the contralateral extremities and can be characterised as positive simple motor responses involving one to three joints. Comparable observations were made when stimulating the cingulate motor area in non-human primates. The characteristics of these motor responses differ from typical SSMA responses which tend to predominately involve proximal extremities and axial posture (Tanji, 1994; Chassagnon et al., 2008). In addition, despite the proximity of electrode contacts in our case, neighbouring sites revealed an upper extremity motor response on the ventral bank and a lower extremity motor response on the dorsal bank of the cingulate sulcus. Stimulation of more dorsal contacts did not reveal any symptoms, excluding the possibility that the adjacent SSMA was being stimulated. Furthermore, precise correlation with anatomical structures was aided by MRI/CT image fusion software and the use of depth electrodes minimised fusion errors due to the lack of cortical shift, often caused by craniotomy and placement of subdural grids. In addition, this case is unique in that it was non-lesional and cortical dysplasia was revealed on pathological investigation, thus distortion of anatomical structures was minimised. Cortical reorganisation, however, cannot be absolutely excluded; an inherent limitation of stimulation studies performed in subjects with any pathology. This case adds to the literature supporting the presence of a cingulate motor area in humans and further delineates the topography of motor representation.
Disclosures
The authors have no conflicts of interest to disclose.
Legends for video sequences Video sequence 1 Stimulation of electrode ED2 at 9 mA resulted in tonic flexion of the right elbow and wrist. Video sequence 2 Stimulation of electrode ED3 at 11 mA resulted in dorsiflexion of the right foot. Key words for video research on www.epilepticdisorders.com Syndrome: focal non-idiopathic frontal (FLE) Etiology: dysplasia (architectural) Phenomenology: tonic posture; right Localization: cingulate gyrus