Epileptic Disorders
MENUDrug-resistant parietal epilepsy: polymorphic ictal semiology does not preclude good post-surgical outcome Volume 17, numéro 1, March 2015
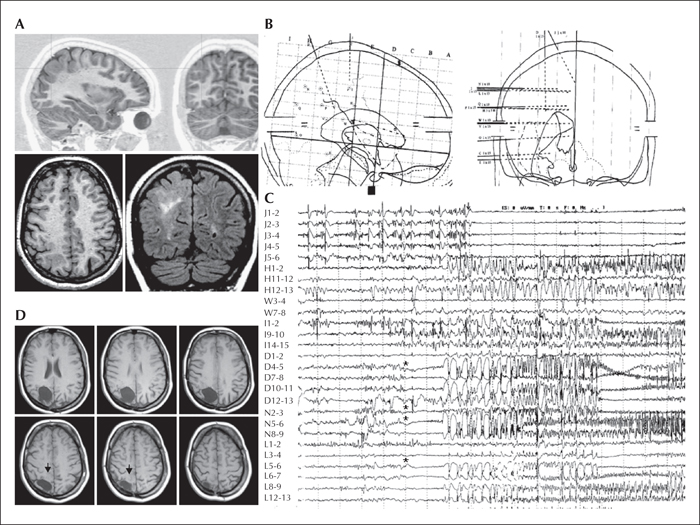
Figure 1
MRI and SEEG of a patient with epilepsy originating from the parietal lobe who was shown to have FCD IIB. The patient had no significant family or personal antecedents. At neurological examination, osteotendineous reflex was slightly prominent in the left, with a moderate deficit of grafognosia in both legs, and a presence of atonic events on the left at Mingazzini I. The patient was right-handed. Epilepsy onset occurred at eight years of age with a generalized seizure, afterwards, seizures were characterized by an initial sensitive-motor sensation of the left foot or, more frequently, an initial impression of “seeing weirdly” (“the ceiling becomes smaller and is moving towards me”), followed by eye deviation to the left (or, sometimes, to the right), flushing, diffuse hypertonia and clonias prevalently to the left, and no apparent post-ictal language deficits. EEG showed: - interictal spike-waves over the right centro-parieto-occipital region; - high-voltage spike or spike-wave on the right parietal region correlating with the negative myoclonus of the left arm (and frequently the left leg); - ictal onset in the right centro-parietal region when associated with the major attacks (one seizure) and in the right centro-temporal region when associated with the visual auras (two seizures).
MRI (A) showed a probable dysplastic lesion in the right centro-parietal cortex in its superior and posterior portion. In order to better delineate the limits of the EZ and its relation with eloquent areas, an SEEG (see stereotactic implantation scheme in [B]) was performed (electrodes and corresponding structures investigated are outlined below) and showed an ictal onset (see seizure trace in [C]) in the superior parietal lobule, namely in the contacts N2-3, N5-6, L6-7, D4-5 (stars), followed by a fast and consistent involvement of the paracentral lobule (electrode J; arrow) and by rhythmic fast activity arising asynchronously from different sites of the dysplastic lesion and spreading to the inferior parietal lobule, precuneus, and cingulate gyrus. Low-frequency cortical stimulations of electrode J induced clonic jerks of the leg and hand, and confirmed that electrode J indeed was implanted in the paracentral lobule. As a consequence of the intracerebral neurophysiological and anatomo-functional findings, an incomplete resection of the dysplastic lesion (arrows in [D]) and epileptogenic zone was performed at 24 years of age. Histology revealed FCD IIB. The patient was classified as Engel class IVa from surgery, despite a vagal stimulator recently implanted. At last follow-up visit (14 years post-surgery), neuropsychological examination showed an amelioration of visual-motor planning abilities.
The cerebral structures investigated by each electrode in the stereotactic scheme (B) are listed from mesial to dorsal surfaces; the numbers of each electrode in the trace montage follow the same direction (1 to 15: mesial to dorsal). P: cingulate gyrus, inferior parietal lobule; H: cingulate gyrus, postcentralgyrus; I: cingulate gyrus, postcentralgyrus; V: cingulate gyrus, supramarginalgyrus; W: supramarginalgyrus; C: para-hippocampal gyrus, hippocampus, middle temporal gyrus; E: fusiform gyrus, inferior temporal gyrus; L: precuneus, lesion, inferior parietal lobule; N: precuneus, superior parietal lobule; O: lingual gyrus, middle occipital gyrus; J: paracentral lobule; D: lesion, superior parietal lobule; Q: cuneus, superior parietal lobule.
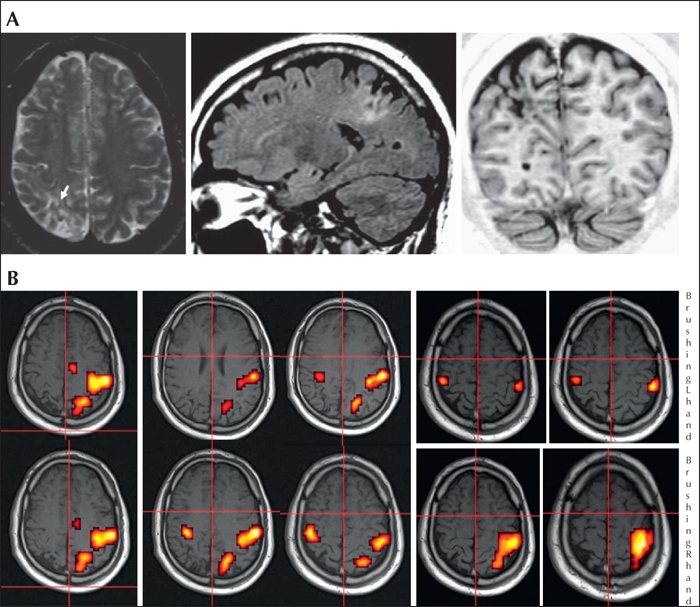
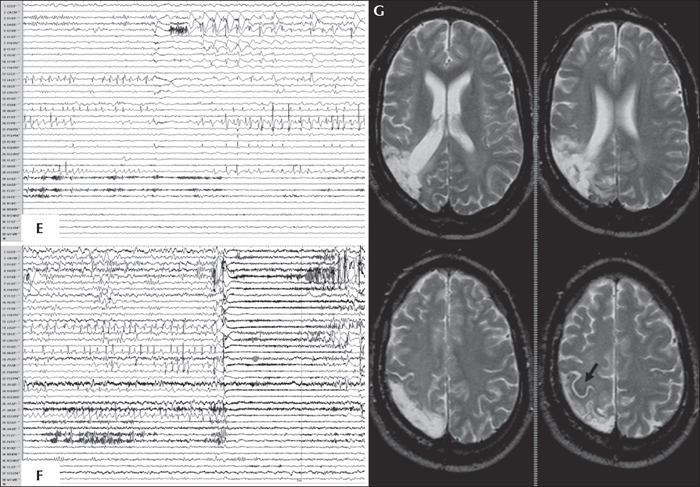
Figure 2
MRI, fMRI and SEEG of a patient with epilepsy originating from the parietal lobe who was shown to have right parietal ulegyria and gliosis. The patient was born before term (34 weeks of gestation) with normal delivery. There were no focal neurological deficits, although agnosia digitorum was present with moderate cognitive impairment involving specific deficits in: problem-solving abilities, verbal fluency, visuo-spatial memory, visuo-motor planning, and facial recognition. The patient was ambidextrous. Epilepsy onset occurred at eight months of age, with annual seizures, frequently facilitated by fever and characterized by diffuse hypertonia and staring or eye-head deviation, as well as constant secondary generalization. At the time of the presurgical evaluation at 31 years of age, the seizures had a monthly frequency, and their semiology consisted of an initial tingling-like sensation localized to the fingers of the left hand with a centripetal direction up to the shoulder, and afterwards towards the left foot, followed by eye and head deviation either to the right (more frequently) or to the left, and left arm hypertonia. Interictal EEG showed spike-waves over T4-T6 and P4-T6 and asynchronous spikes over C4-P4, constantly involving Pz, while ictal EEG showed a right temporo-parietal initial rapid discharge. MRI (A)revealed right parietal ulegyria, involving both the inferior parietal lobule and the post-central gyrus, including the underlying white matter (arrow: Rolandic sulcus). fMRI (B) showed an activation of the left primary motor area, the left SMA, and a left post-central area during the finger-tapping (F-T) task of the right (R) hand; an activation of a left post-central area and the primary motor area, bilaterally, with greater extent and intensity on the left, during finger-tapping of the left (L) hand; an activation of the left somatosensory area during brushing of the right hand; and an activation of two small areas, bilaterally, none of which corresponded to the theoretical position of the somatosensory area in the right post-central gyrus during brushing of the left hand. The activated sensorimotor areas did not appear to be contiguous with the ulegyric lesion.
An SEEG investigation of the right centro-parieto-temporal cortex was performed (see stereotactic scheme in [C]; electrodes and corresponding structures investigated are outlined below) demonstrating the presence of:
subcontinous spike-waves (D) involving the post-central gyrus (X12-13), the intermediate-external aspect of the inferior parietal region (external contacts of R and intermediate-external contacts of P) and the posterior part of precuneus (L4-5), and polyspikes in the fusiform gyrus and inferior temporal gyrus (electrode D), while activity in the precentralgyrus (X8-9) was free of interictal abnormalities; - subclinical paroxysms (E) arising from the inferior parietal area (see fast activity from intermediate-external contacts of electrodes L and P), in the intermediate-external aspect of theparieto-occipital junction (see flattening at electrodes Y and V), but also in the inferior temporal and fusiform gyri (see rhythmic bursts of high-voltage polyspikes at electrode D); - ictal onset, either similar to the subclinical paroxysms or, more frequently, characterized by a high-voltage spike (F) immediately followed by fast activity of very low voltage involving the majority of electrodes, more pronounced in the intermediate contacts of L, D, P and external contacts of V and R.
Electrical cortical stimulations of the supra marginal gyrus and the post-central gyrus induced the habitual aura, and revealed a normal function of the motor area and a partially functional somatosensory area. Invasive neurophysiological and anatomo-functional findings permitted to delineate an epileptogenic zone localized in the intermediate-external part of the parietal region and involving the post-central gyrus in the superior aspect, as far as the parietal-occipital junction in the inferior aspect (G), the total resection of which leaded to complete seizure freedom (Engel class Ia) without motor-sensitive deficits. Post-operative visual field testing revealed a predicted, incomplete left inferior quadrantanopsia. Histopathological analysis revealed the presence of gliosis. Neuropsychological evaluation at five years of follow-up showed an amelioration of verbal fluency, visuo-motor planning, facial recognition, and problem-solving abilities.
The cerebral structures investigated by each electrode in the stereotactic scheme (C) are listed from mesial to dorsal surfaces; the numbers of each electrode in the trace montage follow the same direction (1 to 15: mesial to dorsal). F1-2: anterior part of paracentral lobule; F3-5: superior part of precentralgyrus; G1-3: posterior part of paracentral lobule; G4-7: superior part of postcentralgyrus; H1-6: anterior part of superior parietal lobule; W1-5: transverse temporal gyrus; W6-7: posterior part of superior temporal gyrus; D1-5: intermediate part of fusiform gyrus; D6-11: posterior part of inferior temporal gyrus; N1-2: posterior part of central gyrus; N9-13: intermediate part of precentralgyrus; S1-2: posterior part of central gyrus; S11-15: inferior part of postcentralgyrus; X1-2: posterior part of paracentral lobule; X7-9: intermediate part of precentralgyrus; X11-14: postcentralgyrus (lesion). P1-2: posterior part of central gyrus; P6-11: lesion; P12-15: supramarginalgyrus; R1-2: posterior part of central gyrus; R10-11: lesion; R12-15: supramarginalgyrus; C1-3: intermediate part of hippocampus; C11: posterior part of middle temporal gyrus; L1-4: posterior part of precuneus; L5-7: lesion; L8-11: posterior part of inferior parietal lobule; V1-2: posterior part of precuneus; V9-15: angular gyrus (lesion); Y1-3: anterior part of cuneus; Y5-6: lesion; Y7-10: superior occipital gyrus. 

