Epileptic Disorders
MENUDeterminants of postictal agitation and recovery after tonic-clonic seizures in generalized and focal epilepsy Volume 24, numéro 1, February 2022
Bilateral tonic-clonic seizures (TCS) can occur in patients suffering from focal and generalized epilepsy. This type of seizure is usually followed by a gradually resolving postictal state characterized by unresponsiveness, confusion and amnesia [1]. The postictal state can have a more significant impact on quality of life of patients than the ictus itself [2]. The pathophysiological mechanisms of the postictal state are poorly understood and often, this does not receive much attention in the management of epilepsy [3, 7]. In this study, we reviewed video recordings for postictal manifestations, including agitation, after tonic-clonic seizures in patients with various epilepsy localizations to identify differences and predictive factors.
Materials and methods
After receiving approval from the institutional review board at Vanderbilt University Medical Center, we retrospectively searched the electronic medical records for patients ≥18 years with generalized or focal epilepsy who had at least one tonic-clonic seizure (TCS) recorded in the epilepsy monitoring unit (EMU) between January 2010 and December 2019. The patients were consecutively identified, starting with the most recent, due to greater accessibility. We planned to study 15 patients each in four groups: idiopathic generalized epilepsy (IGE), left temporal lobe epilepsy (LTLE), right temporal lobe epilepsy (RTLE), and frontal lobe epilepsy (FLE). Diagnosis of IGE was established by clinical history and EEG. Diagnosis and localization of focal epilepsy was established by clinical history, EEG and good outcome after epilepsy surgery (seizure-free or rare seizures only after at least one year of follow-up); or by a combination of electro-clinical features and a putative epileptogenic lesion restricted to one lobe identifiable on MRI brain.
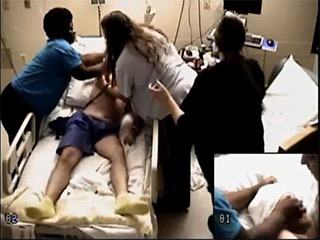
For each patient, the first TCS with reliable ictal and postictal data (video and EEG) was analyzed. A TCS and its various phases were defined according to Theodore et al. [4]. The postictal phase started at the end of clinical seizure activity and ended when the patient started to follow commands and became oriented. The time between onset and end of postictal phase was referred to as “time to responsiveness” (TTR). Postictal agitation was defined and classified as “mild” if the patient demonstrated horizontal movements or rotation of the trunk and pelvis while lying or sitting in bed and/or tried to remove medical equipment (EEG leads, nasal cannula, etc.), but was easily directed to stop that. Postictal agitation was classified as “marked” if patients attempted to get out of bed, were kicking or boxing, tried to remove medical equipment, interfered with nursing care, and were difficult to direct [5]. The standard intervention in our EMU is to rush to the patient's room, ensure patient safety, verify patent airway, suction secretions, administer oxygen, start pulse oximetry, and turn the patient on their side. After the end of the seizure, nurses and technologists attempt to get a response from the patient through tactile and verbal stimulation. However, on rare occasions, seizures were not detected in a timely manner, and were identified on later record review.
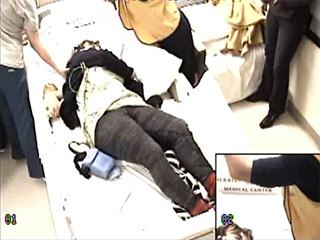
The video and EEG for each selected seizure was analyzed from clinical or EEG onset (whichever was first) until the patient started to follow commands (see video sequences 1, 2 as examples of the videos). For some seizures, recovery had not yet occurred by the end of the video clip; the end of the clip was presumed to be the end of the postictal phase in one preliminary analysis. These seizures were excluded from a second TTR analysis. One board-certified neurophysiologist (AD) reviewed all the electroclinical data and entered de-identified information in a secure online database. A second board-certified neurophysiologist (SZ) who was blinded to the clinical data also reviewed the videos for postictal agitation; differences were resolved by reanalysis in conjunction with input from a third board-certified reviewer (BAK).
The following variables were collected for each patient: current age, gender, age at seizure onset, history of psychiatric illness (mood disorder/anxiety disorder), location of MRI brain lesion (if present), history of resective epilepsy surgery (focal epilepsy), pathological diagnosis, outcome at one-year of follow-up, ictal characteristics (ictal EEG findings, clinical onset, EEG onset, duration of focal phase, duration of tonic-clonic phase, clinical seizure end, EEG seizure end), and postictal characteristics. Postictal characteristics included presence of postictal generalized EEG suppression (PGES), duration of PGES, duration of postictal video analyzed, postictal agitation, duration of postictal agitation, postictal snoring, ictal/postictal oxygen administration, and postictal suctioning.
Descriptive statistics were used to report demographic and clinical data. Groups were compared using Fisher's exact test, Chi-squared test, and one-way ANOVA. Paired comparisons were performed using Mann-Whitney U statistic. Spearman's rank correlation coefficient was used to determine relationship between groups. A p value of 0.05 was considered statistically significant.
Results
We reviewed 60 TCS in 15 patients with IGE and 45 patients with focal epilepsy (15 RTLE, 15 LTLE, 15 FLE). Forty-one patients with focal epilepsy underwent epilepsy surgery after video-EEG and had Engel Class I to IIA outcome at one year, including all TLE patients and 11 FLE patients. Four patients with FLE did not have surgery, but had a putative epileptogenic lesion in the frontal lobe (right superior frontal gyrus encephalomalacia with surrounding hyperintense signal; left frontal encephalomalacia with associated hemosiderin deposition; gray matter heterotopia in the left frontal lobe; posterior right frontal low-grade neoplasm) and neurophysiological data that supported frontal localization.
The mean age of the patients was 36.8 years (range: 19-62) and 34 were female. Other patient characteristics are reported in table 1. Postictal video was analyzed for a mean duration of 11.5 minutes (range: 3.5-30 minutes) for each seizure in all 60 patients. The return of postictal responsiveness could not be determined in 32 patients despite analyzing 11.4 minutes (mean) of video per seizure as the videos were clipped prior to clinical recovery. This was done to reduce storage space on computer servers at our institution. For the subgroup of 28 patients (46.6%) in whom recovery had occurred before the end of the video clip, postictal responsiveness was observed after a mean duration of 11.6 minutes (range: 4.3-29 minutes).
The average total seizure duration was 73 seconds (range: 40-100) for generalized-onset and 148.7 seconds (range: 52-762) for focal-onset seizures (not significant). Total seizure duration was longest (mean: 219.6; range: 75-762 seconds) in the LTLE group (p=0.006). The duration of the tonic-clonic phase was relatively similar in all four groups, though longest in the RTLE group (not significant). Other ictal and postictal characteristics are reported in table 2.
Agitation was observed in 14 of 60 patients (23.3%). This was “marked” in one patient (RTLE), and “mild” in 13. Inter-rater reliability for identifying agitation between two reviewers (one of whom was blinded) was near perfect (Kappa statistic: 0.95). Agitation was most common in the LTLE group (7 of 15) followed by FLE (4 of 15), RTLE (2 of 15), and least commonly observed in the IGE group (1 of 15). The differences were significant only between IGE and LTLE groups (p=0.035; Fisher's exact test). Among the seven LTLE patients with agitation, language dominance was left sided in five (documented by Wada test in four and postictal aphasia in one) and bilateral in two (documented by Wada test in one and functional MRI in one). There was no postictal agitation in three LTLE patients with right hemisphere language dominance. The mean duration of agitation was 1.8 minutes (range: 0.2-3.4 minutes). Postictal agitation did not differ statistically by gender, history of psychiatric illness, or presence of PGES. There was no relationship between postictal agitation and total seizure duration, duration of bilateral tonic-clonic phase, or duration of PGES. Postictal agitation was not less likely with oxygen administration (performed in 56 patients) and suctioning (performed in 49 patients). There was no correlation between duration of postictal agitation and duration of seizure, tonic-clonic phase, or PGES (Spearman's Rho).
The one patient with marked agitation was a 47-year-old man with seizure onset at age 18. He had loss of brain substance in the inferior right temporal lobe cortex and subcortical white matter on brain MRI. He underwent right anterior temporal lobectomy and was found to have mesial temporal sclerosis on pathological examination. He was free of seizures and auras at one year of follow-up.
Based on analysis of the whole patient group (n= 60) in which TTR was estimated if the video clip ended before recovery, TTR was shortest in the IGE group (mean: 8.4 minutes), followed by RTLE (mean: 10.2 minutes), FLE (mean: 10.7 minutes) and LTLE (mean: 12.9 minutes); these differences were not statistically significant. No further analyses were performed for TTR in the whole group. Based on subgroup analysis of patients for whom postictal responsiveness was determined with certainty (n=28), TTR was shortest in the FLE group (6.6 minutes), followed by IGE (7.2 minutes), RTLE (10 minutes), LTLE (15.7 minutes) groups (p=0.02) (seetable 3 for details). Pairwise differences in TTR were statistically significant for FLE vs. LTLE and IGE vs. LTLE. Time to responsiveness was longer in patients with agitation than without agitation (13.9 minutes vs. 7.7 minutes; p=0.048). There was no correlation between TTR and duration of seizure, duration of tonic-clonic phase, or duration of PGES (Spearman's Rho).
Discussion
We found that although postictal agitation occurred after almost a quarter of TCS, marked agitation was rarely encountered. Postictal agitation was related to the location of the epileptogenic zone; it was more likely after left temporal-onset seizures and least likely after generalized-onset TCS. Agitation occurred after the immediate postictal coma but prior to postictal recovery. In all patients with agitation, it occurred within 30 minutes after the seizure ended, referred to as T1 phase of the postictal state [6]. There was no relationship between agitation and patient characteristics, such as gender or history of psychiatric illness; ictal characteristics, such as total duration of seizure, duration of tonic-clonic phase; postictal characteristics, such as presence or duration of PGES; or standardized peri-ictal interventions, such as oxygen administration and oral suctioning. The relationship between postictal agitation and epilepsy localization found in this study suggests that ictal-postictal dysfunction in the language network may play a role in its occurrence. This hypothesis is supported by left language dominance in five of the seven LTLE patients with agitation. The remaining two had bilateral language representation. One possible mechanism could be frustration over impaired communication in the presence of impaired sensorium postictally.
Similar to postictal agitation, TTR was independent of ictal and postictal variables, such as duration of seizure, tonic-clonic phase, or PGES. Interestingly, TTR was significantly longer in patients with agitation than without agitation. The presence of agitation in the postictal period may alert clinicians and caregivers to take extra precautions, as recovery may be longer in such patients. We did not find any relationship between postictal agitation and standard peri-ictal and postictal interventions in the EMU, such as oxygen administration and oral suctioning, although this cannot be determined with certainty since the vast majority of patients received this intervention. Pending further evidence, close clinical monitoring in the post-ictal period may be the best available intervention to mitigate patient harm. Counseling patients and caregivers in this regard may be prudent in appropriate situations. Although we found that agitation was usually “mild” when it occurred, patient behavior such as attempts to remove EEG leads may become more clinically consequential when they have intracranially implanted electrodes. Clinical monitoring becomes even more important in such cases.
We found that postictal recovery (TTR) was significantly more delayed in the LTLE group in comparison to FLE and IGE groups. Comparison of the postictal phase between various seizure localizations has not been reported in TCS. The postictal state has been mainly studied in seizures that did not evolve to bilateral tonic-clonic activity [7]. Interestingly, dominant temporal lobe seizures were associated with longer time to postictal recovery than non-dominant temporal focal impaired awareness seizures [8], and those of frontal lobe onset were found to have a short postictal phase [9].
The study cohort was representative of various localizations of epilepsy validated by video-EEG, and in the case of focal epilepsy, by good outcome after resective epilepsy surgery in all TLE and most FLE patients, or video-EEG data congruent with localization of an epileptogenic lesion in a minority of FLE patients. However, this study is retrospective in nature, and includes a small number of patients, particularly in the TTR analysis. We assessed TTR in a smaller group of patients (n=28) than initially intended (n=60), because the remaining patients had not fully recovered by the end of the available video segment. Future prospective studies can overcome this barrier. The TTR may be influenced by the verbal nature of stimuli and patient responses; this may partially explain why TTR was prolonged in LTLE patients, but cannot explain the greater agitation in LTLE patients. Finally, we evaluated only one TCS per patient. Future studies may evaluate consistency of these findings in patients with multiple TCS.
Supplementary material
Summary slides accompanying the manuscript are available at www.epilepticdisorders.com.
Disclosures
We do not report any financial or conflict of interest disclosures pertinent to this study.
* The results of this study were presented in abstract format at the annual meeting of the American Epilepsy Society in 2020.