Epileptic Disorders
MENUClinical management of voice and breathing problems in two patients with vagus nerve stimulation therapy Volume 23, numéro 1, February 2021
Vagus nerve stimulation (VNS) has been available as a treatment option for refractory epilepsy for more than 20 years with well-established efficacy; at least 50% of patients experience a 50-60% reduction in seizure frequency [1]. Although VNS therapy is generally well tolerated, the known adverse effects, such as vocal cord palsy, cough, throat pain, dysphagia and breathing and voice disorders, can be distressing [2-4]. These laryngeal side effects are induced by either implantation surgery-related trauma to the vagus nerve or electrical stimulation via the vagus and recurrent laryngeal nerve to the larynx and pharynx [5].
The implantation can cause transient or permanent vocal cord paralysis and occurrence varies extensively, from 5% to 79% [6-8]. Most adverse effects are reported to occur when the stimulator is delivering a pulse, and they are often noted to be proportional to the duration, frequency and current amplitude of the stimulation (e.g. see references [9, 10]). The electrical impulse can cause spasmodic contraction of the ipsilateral intrinsic laryngeal muscles, presenting as an immobile left vocal fold in the paramedian to median position during stimulation, usually with a higher stimulation energy [3, 11]. However, a correlation between stimulation parameters and different visible laryngeal patterns or audible voice change is not always evident [3, 6].
Most patients rapidly habituate to the symptoms and reprogramming of the parameters often results in a satisfactory outcome even in those patients whose symptoms persist (e.g. see references [9, 10]).
This study describes two cases illustrating the diversity of the clinical presentation and therapeutic implications of laryngeal side effects of VNS. The patients received VNS treatment for their refractory epilepsy in the neurological outpatient clinic of Tampere University Hospital in 2018-2019. The multidisciplinary assessment and details of the side effects are discussed.
Case studies
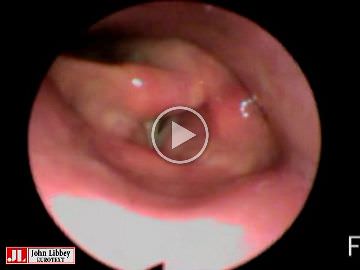
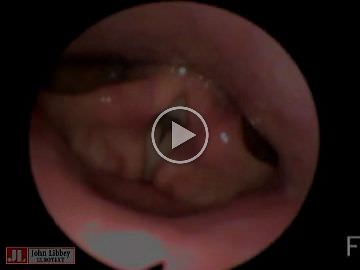
The evaluation of voice and breathing, including flexible fibreoptic endoscopy, were performed by two specialist speech-language pathologists (SA, TM) who assessed the signs and symptoms before and during initial VNS device activation and four times during the follow-up periods of 22 and 13 months (tables 1 and 2). The assessment included self-evaluation of breathing and voice by systematic interview and the VHI (Voice Handicap Index [12]). Further, the voice quality was evaluated perceptually for Patient 1. For Patient 2, it was possible to conduct acoustic voice analyses using AVQI (Acoustic Voice Quality Index 03.01 [13]), because adequate audio recordings were available.
A specialist nurse (SH) programmed the stimulator parameters and monitored the patient with frequent out-patient visits. The neurologist devised the individual care plan (JP).
In both patients, antiepileptic medication was not changed during follow-up (tables 1 and 2). The VNS output current was increased weekly with 0.25 mA increments from the time of activation. Frequency (20 Hz) and pulse width (250 μs) were held constant from the onset of stimulation.
Case 1
Patient 1 was a 51-year-old, passionate amateur athlete with a 17-year history of refractory epilepsy. The aetiology of his epilepsy was right hemisphere temporo-parietal cavernoma, which was resected without any meaningful effect on seizure frequency or severity. The patient was implanted with a VNS AspireSR® M106, manufactured by LivaNova PLC. Post-operatively, the patient experienced hoarseness, inability to shout and laboured breathing during exercise.
After the implantation, the patient had left vocal cord paralysis, causing breathing and voice dysfunction. His maximum output current (1.75 mA/magnet 2.0 mA) was reached three months post-operation. At seven months, the quality of his voice was better when the stimulator was active. At 10 months, his breathing was still troubled during exercise. He had gained some movement in the paralysed vocal fold, and therefore his voice was now more normal when the stimulation was off and hoarser when the stimulator was firing. At 11 months, the patient's breathing difficulties occurred only during high-intensity exercise, and he exhibited only transient stimulus-related dysphonia. At 22 months, the patient's breathing and voice symptoms remained stable. He felt that he could manage with his voice and he changed his exercise routine. He now avoided very-high-intensity exercise and sports, such as swimming, which were too demanding for his breathing capacity. At this point, the patient had a Class IIIA response (<50% reduction in seizure frequency but improved ictal severity) to VNS therapy [14].
Case 2
Patient 2 was a 40-year-old journalist with refractory temporal epilepsy since childhood. He was not a candidate for resective surgery because of his inoperable right-sided cortical occipitotemporal dysplasia. He was implanted with a VNS SenTivaTM M1000, manufactured by LivaNova PLC. After the operation, the patient experienced difficulties when speaking; his voice was hoarse, whispery and easily broken. About two weeks after the implantation, he also began to experience pain in the neck area.
The patient had left vocal cord paralysis due to the implantation, causing him subjective swallowing difficulties and mild dysphonia (table 2). Operation-related symptoms recovered spontaneously within three months although minimal dysfunction in the left vocal cord persisted for six months.
At two to three months after implantation, 1.25 mA stimulation current was associated with breathing and voice problems. The patient experienced mild dysphonia, but the acoustic analyses were within normal range. The endoscopy revealed abnormal left vocal cord function with OFF-stimulation and vocal cord spasm with 1.25 mA stimulation. The stimulation with 1.50 mA again provoked a stronger vocal cord spasm, which restricted his breathing and induced significant voice dysfunction.
At six months, he still had stimulus-related swallowing and breathing discomfort. The higher the stimulation energy, the worse were his laryngeal problems. In particular, the unpredictability of his voice disturbed him.
At eight months, the stimulation of 1.25 mA still caused discomfort and hampered his work, so the current was tapered down to 1.0 mA for daytime use (10am to 6pm), whereas at night (6pm to 10am), the current remained at 1.25 mA. In order to intensify the VNS therapy, the OFF-time for the night cycle was decreased to 3 minutes, whereas in the daytime, OFF-time was kept at 5 minutes. The patient was satisfied; the discomfort and the voice changes were more subtle. At 10 months, the discomfort and the voice changes were more subtle in the day-time, but there were no signs of adaptation to the night-time current of 1.25 mA or the magnet current 1.50 mA. Finally, at 13 months, the patient coped with the minor stimulation-related voice changes. According to the acoustic analyses, he had also adapted to the 1.25 mA current, which no longer caused him dysphonic hoarseness. The patient had a Class IIB response (more than 50% reduction in seizure frequency but without any change in ictal or post-ictal severity) to VNS therapy [14].
Discussion
Both operation and stimulation related side effects occurred in these patients and lasted for several months. Recurrent laryngeal nerve paralysis-related vocal cord hypofunction and stimulation-related vocal fold spasm accounted for the adverse effects, but the experienced voice and breathing problems and the adaptation to stimulation were distinctive.
Patient 1 was an enthusiastic athlete; thus, breathing difficulties restricted his performance in sports. His breathing gradually eased along with recovery of his vocal cord paralysis and adaptation to stimulation. However, some difficulty remained. The VNS parameters were not radically changed because of two main reasons. First, the effect on seizure control was modest, and lowering the overall stimulation energy was not considered appropriate. Second, at some point, the stimulation energy was useful for the paralysed left vocal cord. In retrospect, it could have been beneficial to customize an “exercise setting” for him, for example, by lowering the current for a couple of hours during the day. Nevertheless, his devise, AspireSR® M106, does not include this option. Eventually, he started to avoid the most demanding sports as a compensatory strategy.
Because the implantation had evoked vocal cord paralysis, his voice at first improved while the stimulator was firing. Later, when the paralysis recovered to some extent, the stimulation caused his voice to worsen; stimulation is known to tend to trigger spasms in functioning vocal cords [3]. To our knowledge, similar cases on which stimulation temporarily benefitted voice production have not been documented before. However, the phenomenon is understandable as indirect or direct neuromuscular electrical stimulation may be effective as an adjunctive treatment for some cases of pharyngo-laryngeal dysfunction [15].
Patient 2, the journalist, suffered from poor voice quality. For several months, he experienced significant voice problems, especially at higher currents. Unlike most previously published cases (for example, see [9, 10]), his adaptation to the stimulation currents took much longer. During the follow-up, the patient's VNS parameters were adjusted in order to enhance his performance at work, especially when talking on the phone. Because his VNS device (SenTivaTM M1000) enabled settings to be customised, it was possible to decrease the current during working hours in daytime while maintaining a higher current and shorter stimulation OFF-time at night. In this way, the patient could use his voice during working hours, but the overall therapeutic effect for his seizures was not compromised.
These two cases are good examples of how, in some individuals, even seemingly mild voice and breathing problems may impair quality of life. Therefore, specific and up-to-date knowledge of VNS complications is required to ensure good individualized care.
Currently, there are no established assessment protocols for voice and breathing problems of VNS patients. More systematic research is needed to determine the most sensitive assessment tools and the best care practices. However, it is important to at least differentiate patients with vocal fold paralysis from those with only stimulation-related side effects. We recommend using multiple assessment methods, including systematic self-evaluation such as VHI, acoustic or perceptual analysis of voice and endoscopy or even stroboscopy, to enhance investigation of laryngeal functions. We suggest using AVQI as an acoustic measure. AVQI relies on the detection of hoarseness as an indicator of overall voice quality and is therefore well suited for a population whose voice disorders are mainly classified as hoarse, due to vocal cord paralysis or unilateral vocal cord dysfunction. In the future, it may also be possible to record VNS-induced laryngeal motor evoked potentials, as suggested by Vespa et al.[16].
Considering the recommendations for the management of laryngeal problems, there is no definite indication for delaying the increase of output current due to vocal cord paralysis. It is possible that, at therapeutic levels, the stimulation may protect paralysed vocal cords from atrophy [6] and assist in regaining function. The spectrum of suitable VNS candidates is expanding with development in care. In VNS patients, whose occupation, hobbies or social life require intact voice control or who want to undertake strenuous physical exercise, the capabilities of the new VNS devices, enabling customized stimulation cycles, should be acknowledged and exploited.
Disclosures
S. Alantie, T. Makkonen and S. Hietala have received speaker honoraria from LivaNova. J. Peltola has participated in clinical trials for Eisai, UCB, and Bial; received research grants from Eisai, Medtronic, UCB, and LivaNova; received speaker honoraria from LivaNova, Eisai, Medtronic, Orion Pharma, and UCB; received support for travel to congresses from LivaNova, Eisai, Medtronic, and UCB; and participated in advisory boards for LivaNova, Eisai, Medtronic, UCB, and Pfizer.