Epileptic Disorders
MENUBerardinelli-Seip syndrome and progressive myoclonus epilepsy Volume 21, numéro 1, February 2019
- Mots-clés : lipodystrophy type 2, Berardinelli-Seip syndrome, BSCL2, progressive myoclonus epilepsy, neurodegenerative encephalopathy, EEG, vagus nerve stimulator
- DOI : 10.1684/epd.2019.1038
- Page(s) : 117-21
- Année de parution : 2019
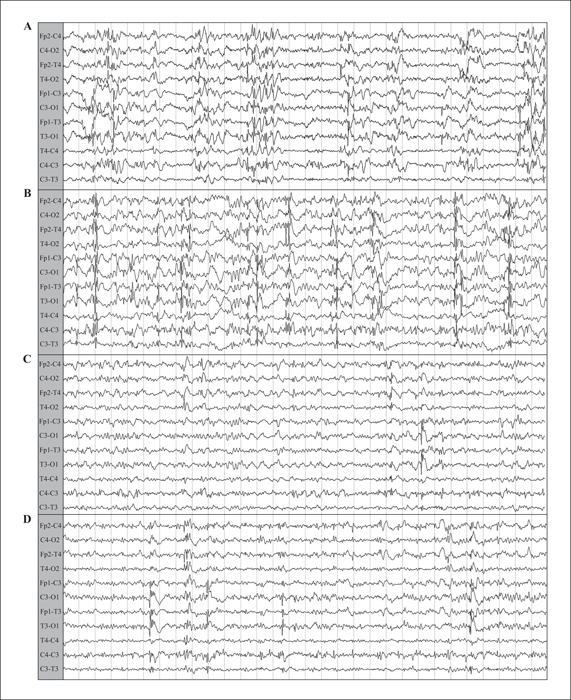
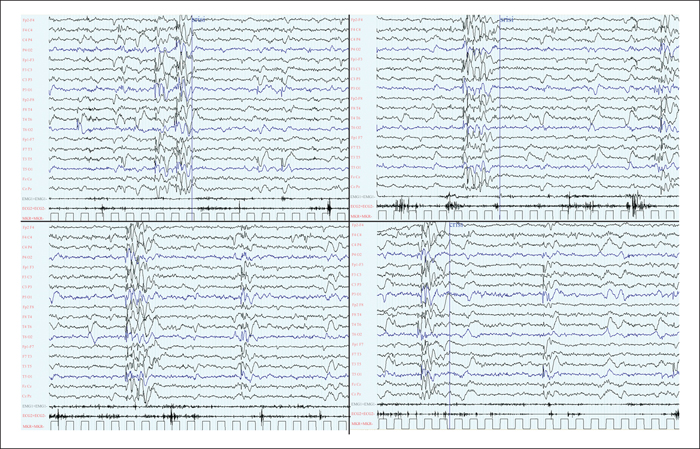
Berardinelli-Seip syndrome, or congenital generalized lipodystrophy type 2 (CGL2), is characterized by a lack of subcutaneous adipose tissue and precocious metabolic syndrome with insulin resistance, resulting in diabetes, dyslipidaemia, hepatic steatosis, cardiomyopathy, and acanthosis nigricans. Most reported mutations are associated with mild, non-progressive neurological impairment. We describe the clinical and EEG data of a patient with progressive myoclonus epilepsy (PME), CGL2, and progressive neurological impairment, carrying a homozygous BSCL2 nonsense mutation. The patient had epilepsy onset at the age of two, characterized by monthly generalized tonic-clonic seizures. By the age of three, he presented with drug-resistant ongoing myoclonic absence seizures, photosensitivity, progressive neurological degeneration, and moderate cognitive delay. Molecular analysis of the BSCL2 gene yielded a homozygous c.(1076dupC) p.(Glu360*) mutation. Application of a vagus nerve stimulator led to temporary improvement in seizure frequency, general neurological condition, and EEG background activity. Specific BSCL2 mutations may lead to a peculiar CGL2 phenotype characterized by PME and progressive neurodegeneration. Application of a vagus nerve stimulator, rarely used for PMEs, may prove beneficial, if only temporarily, for both seizure frequency and general neurological condition.