Epileptic Disorders
MENUAltered vascular permeability but not angiogenesis may play a role in the epileptogenesis of human hippocampal sclerosis Volume 23, numéro 3, June 2021
Hippocampal sclerosis (HS) is the commonest pathology of surgically remediable drug-resistant epilepsy in most series. Histological findings have been well described since 1880 by Wilhelm Sommer and are characterised by loss of pyramidal neurons in the cornu ammonis with corresponding gliosis and shrinkage of the hippocampus [1]. The treatment of epilepsy with anti-seizure medication is largely symptomatic and does little to prevent the intricate process of tissue remodelling occurring in the sclerotic hippocampus. Removal of the sclerotic hippocampus has for a long time been the standard of care for drug-resistant temporal lobe epilepsy. Dissecting the molecular mechanisms underlying epileptogenesis could aid in development of novel disease-modifying agents in order to arrest or delay progression of disease.
Though the pathology is well described, the exact pathogenesis of HS remains poorly understood, including the intriguing question regarding the basis for the striking regional selectivity of neuronal loss. Two of the earliest theories put forward in the early 20th century included the famous Vogt's ‘Pathoklise theory’ and Spielmeyer's ‘vascular theory’. The Pathoklise theory attributed the difference in susceptibility between various sectors to the physicochemical structure of the neurons. Spielmeyer's vascular theory ascribed the selective vulnerability to anoxic/ ischemic damage due the precarious vascular supply and highlighted the relationship of the hippocampal neurons to the vascular territory [2, 3]. However, contrary to the belief that ictus leads to ischemia and decreased vascular supply, an increase in blood flow has been conclusively demonstrated by MR perfusion studies [4].
Angiogenesis is known to play a role in repair following any form of insult or injury to the brain. Mediated by vascular endothelial growth factor (VEGF), it can create an unfavourable hyperexcitable environment that can facilitate epileptogenesis [5, 6]. Angiogenesis and epileptogenesis can result in a self-perpetuating vicious circle. Blocking angiogenesis may therefore provide a therapeutic target in the treatment of drug-resistant epilepsy (DRE) [7].
The role of angiogenesis in HS has not received much attention. Most published studies focussing on angiogenesis have been undertaken using animal models of epilepsy. Pilocarpine-induced status epilepticus in rat models has revealed an increase in the number, length and diameter of blood vessels, along with a leaky blood-brain barrier (BBB) [8]. To date, we found only one study in the published literature that has evaluated the occurrence of angiogenesis in human hippocampi, in cases of temporal lobe epilepsy (TLE), reporting an increase in vascular density in HS [9]. However, none of the studies have taken into consideration hippocampal volume loss that occurs in HS, which could be a confounding factor for the assessment of vascular density. The present study was designed to address these lacunae. We aimed to determine the extent of angiogenesis in resected specimens of human HS and correlate this with hippocampal volumetry assessed by MRI. Further, we evaluated the role of vascular permeability in the pathogenesis of DRE.
Our centre is a tertiary referral centre in South India catering exclusively to patients with neurological and neurosurgical disorders. It has an advanced comprehensive epilepsy program running for more than two decades. Hence, we retrieved all operated cases of HS in order to determine the role of angiogenesis in DRE due to HS. To the best of our knowledge, this is the single largest study to evaluate angiogenesis in human hippocampi from cases of HS.
Materials and methods
Consecutive patients with drug-resistant temporal lobe epilepsy, undergoing resective surgery for HS, who consented to participate in the SATYAM, DST project between 2015-2017, and who agreed that their tissues could be used for the study, were included. The study group included 30 histologically confirmed cases of HS and 30 age-matched controls selected from cases who succumbed to road traffic accidents without hippocampal injury or history of seizures (post-mortem delay: 4.5 hours – 24 hours). Clinical and demographic data including age at onset of habitual seizures, seizure semiology, duration and frequency of seizures, history of febrile seizures in childhood and post-surgery Engel's outcome with duration of follow-up were reviewed (Authors 4 and 5) (supplementary table 1) Neuroimaging features of all the cases were reviewed (Author 3) and cases with focal lesions were excluded. The study was approved by the Institute Ethics Committee.
Hippocampal volumetry (voxel-based morphometry)
Hippocampal volumetry was calculated using voxel-based morphometry. The image processing was performed on Statistical Parametric Mapping 5 software (SPM5) using VBM tools 5.1 toolbox (Welcome Department of Imaging Neuroscience, London; http://www.fil.ion.ucl.ac.uk/spm). VBM5 uses a unified segmentation approach that integrates image registration, MRI inhomogeneity bias correction, and tissue classification [10, 11].
Immunohistochemistry
Four-micron thick serial sections were cut from formalin-fixed paraffin-embedded blocks from the mid body of the hippocampus. Indirect immunohistochemistry (IHC) was performed using the Ventana Benchmark automated staining system (Ventana Benchmark-XT). Briefly, following deparaffinization, heat-induced epitope retrieval of antigen was performed followed by endogenous peroxide blocking. Incubation with primary antibody was followed by adding a universal cocktail of secondary antibody linked to horseradish peroxidase. Sections were stained with 3,3-diaminobenzidine and counterstained with haematoxylin, dehydrated, cleared and mounted. The primary antibodies used were anti-NeuN (1:500, A60, Merck Millipore, USA), anti-GFAP (1:200, 6F2, Dako, USA), anti-CD31 (1:100, JC/70A, Thermofisher, USA), anti-CD105 (1:50, polyclonal, Thermofisher, USA), anti-VEGF (1:200, VG1, Thermofisher, USA), anti-IgG (Ready to use, anti-human HRP conjugated, GeNei, India), anti-human serum albumin (1:1500, polyclonal, abcam, USA) and anti-aquaporin 4 (1:10000, polyclonal, Sigma, USA).
Quantification of vascular density
CD31-labelled slides were digitised (using Leica SCN400). ImageJ software (NIH, version 1.52a) was used for counting the microvessels and calculating the area of the hippocampus. Vessels in each CA sector were counted individually. Vascular density per mm2 was calculated as total number of blood vessels/area of hippocampus. CD105, a transmembrane glycoprotein expressed on vascular endothelial cells during active angiogenesis, was used to count newly formed vessels.
Semiquantitative grading
The percentage of neuronal loss in each sector was graded based on histochemistry for NeuN and cases were classified as per the consensus classification of the International League Against Epilepsy (ILAE) 2013, into HS type 1, 2, 3 and no HS/ gliosis [12]. IgG and albumin IHC were used to label the exuded plasma, to assess BBB permeability. This was graded based on the number of vessels showing perivascular neuropil staining within the hippocampus: score of 0=no perivascular labelling; score of 1=perivascular staining of neuropil around one vessel; score of 2=perivascular staining of neuropil around two vessels; score of 3=perivascular staining of neuropil around three or more vessels.
Expression pattern
IHC for VEGF and AQP4 were not graded and only the expression pattern between the cases and controls were evaluated and compared.
Statistical analysis
The data were analysed using SPSS version 22. The Shapiro-Wilk test of normality was performed and based on the findings, appropriate parametric or non-parametric tests were used. Independent sample t-test and ANCOVA was used to compare cases and controls. Pearson correlation, Spearman's Rank correlation, Kendall tau Rank correlation and Partial correlation was used to assess the relationship between variables. The significance level was set at p< 0.05.
Results
Clinical characteristics
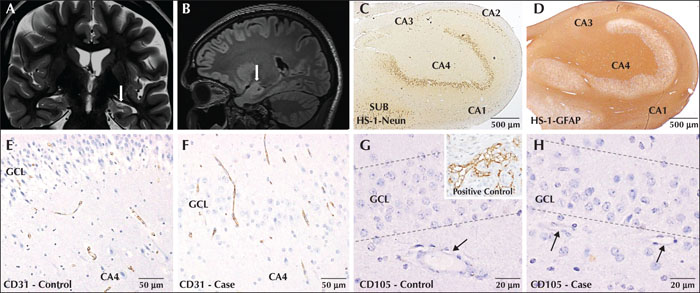
The majority of the patients with HS were in the age group of 21 to 30 years. The cohort was age matched, but gender matching could not be done (due to non-availability of tissues as road traffic accident victims were more frequently males). The mean age at onset of seizures was 10.8 years (range: 0.3–32 years). The mean duration of seizures was 16 years (range: 2–32 years). All the cases presented with focal impaired awareness seizures with focal to bilateral tonic-clonic seizures. The frequency of seizures just before surgery ranged from one episode per day to an occurrence of less than one episode per three months. A score of 1 to 5 was allotted by us to grade the frequency of seizures, with a score of 5 indicating highest frequency. Post-surgery clinical outcome was available in 27/30 cases with a minimum of one year of follow-up. All but one, were completely seizure-free since surgery with Engel Class IA outcome. The single case with Engel Class IC outcome had one seizure episode at two months post-surgery. Six patients had a history of febrile seizures in childhood (20%). As per the ILAE 2013 classification for HS, 90% were classified as HS type 1 (27/30), 6.7% HS type 2 (2/30) and 3.3% HS type 3 (1/30) (figure 1A-D).
Vascular density between HS and controls
CD31 was used to quantify microvascular density (figure 1E, F). Independent sample t-test showed significantly higher mean vascular density in HS (8.71/mm2) compared to controls (7.94/mm2) (p<0.05) and significantly lower mean area of the hippocampus in HS (21.11 mm2) compared to controls (28.35 mm2) (p<0.0001). Since vascular density is dependent on area, ANCOVA was used to determine the difference in estimated means, controlling for the effect of covariate (area). Estimated mean vascular density by ANCOVA did not show any statistically significant difference between the cases (8.34/mm2) and controls (8.33/mm2) (p>0.05). Hence the increase in vasculature may be relative to the decrease in area of the hippocampus in cases with HS relative to controls.
Vascular density and hippocampal volume in HS
Pearson correlation showed a negative correlation between vascular density and hippocampal volume; the higher the vascular density, the lower the hippocampal volume (p<0.05). Partial correlation, on the other hand, which measures the degree of association between two variables, while controlling for the effect of an additional variable (i.e. area of hippocampus), did not show significant association between vascular density and hippocampal volume (p>0.05). This indicates that when the confounding effect of the area of hippocampus was eliminated, there was no correlation between vascular density and hippocampal volume, confirming that the increase in vascular density was an apparent effect caused by shrinkage of the hippocampus.
Angiogenesis
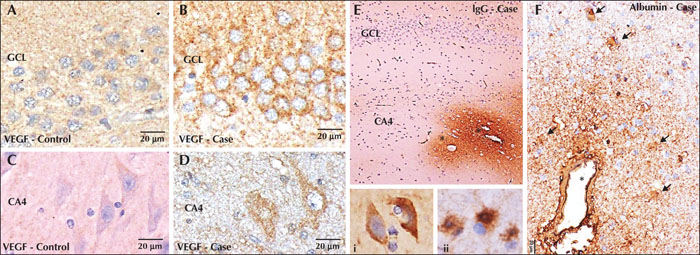
CD105, a marker expressed by endothelial cells of newly formed blood vessels, did not label any vessel, in either HS cases or controls, indicating an absence of angiogenesis (figure 1G, H). Increased expression of VEGF was seen in the granule and pyramidal neurons in cases compared to controls, which showed no to minimal basal labelling (figure 2A-D).
Blood-brain barrier integrity and membrane water channels
Positive staining was observed for IgG and albumin in HS cases in the exuded plasma and the perivascular neuropil, with a concentration gradient, with strong labelling in the vessels in the hippocampus and subiculum. Within these regions, the neurons showed uptake of IgG and albumin within their cytoplasm. Similarly, the astrocytes in the vicinity of these zones also revealed uptake of IgG and albumin within their cell bodies and during hyperplastic processes (figure 2E, F). Controls showed no labelling or mild positivity of the vessels, without any perivascular labelling of neuropil. The IHC pattern of staining and scoring for albumin and IgG was very similar (for details, refer to the supplementary material). Based on the semiquantitative scoring, the Kendall tau test did not show any correlation between IgG or albumin and vascular density or hippocampal volume. This suggests that microvascular leakage is not associated with alterations in microvessel density.
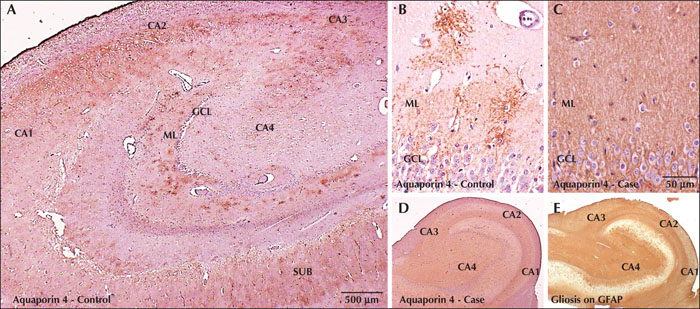
AQP4 IHC in controls revealed a punctate pattern of labelling along the stellate astrocytic processes surrounding blood vessels. This pattern of labelling was most prominent within the molecular layer of the dentate, followed by CA3, CA4, CA2 and least in CA1, and appeared to correspond to the synaptic density within each region (figure 3A, B). In cases with HS, the pattern of distribution and the punctate type of labelling was lost, and instead there was diffuse homogenous AQP4 immunoreactivity with staining of neuropil throughout the Ammons horn (figure 3C, D). This homogeneous and intense immunoreactivity paralleled the pattern of dense gliosis seen within the sclerosed hippocampi demonstrated by immunohistochemistry for GFAP (figure 3E).
Correlation with clinical parameters
Spearman's Rank correlation did not show any significant correlation between the clinical parameters such as age at onset of seizures, duration of seizures or frequency of seizures and either vascular density, hippocampal volume or IgG/ albumin score.
Discussion
This is the largest study to evaluate angiogenesis and vascular permeability in HS, and the only study that has attempted to correlate vascular density and hippocampal volumetry assessed by MRI (table 1 table 1).
We evaluated microvascular density in HS and failed to detect any difference in the vascular density between HS and controls. This is in contrast to the study by Raigu et al., who documented increased vascular density in HS compared to controls. In their study, vascular density was evaluated in three regions (CA1/2, CA3/4 and DG) over a fixed area (total field: 0.08mm2) and data were pooled to obtain a mean value for the whole hippocampus and compared with controls [9]. No correction was applied for the shrinkage and volume loss of hippocampus in HS, which introduces a confounding bias. In our study, we evaluated the vascular density in all the subfields and applied statistical correction to normalise the area between HS and controls. Without the statistical correction, greater microvascular density was found in HS compared to controls, similar to the results of Raigu et al[9]. However, on adjusting for the area, no difference in vascular density was found, indicating that the increase in vascular density was an apparent increase induced by reduction in volume of the hippocampus in HS, rather than true angiogenesis. This is also corroborated by the fact that the marker for neoangiogenesis (CD105) was negative in our study. Raigu et al. also included TLE cases with conditions other than HS, such as tumours, vascular malformations, and ischaemia [9].
Unlike our study, Boldrini et al. noted an age-related decline in angiogenesis in normal hippocampal tissues [13]. The authors used nestin expression as a marker for angiogenesis, although nestin is also expressed in the mature CNS vasculature in endothelial cells, pericytes and periendothelial cells as well as reactive astrocytes [14, 15]. Hence the age-related decline seen by Boldrini et al. could simply reflect the decrease in vascular density of pre-existing vasculature highlighted by nestin rather than newly formed vasculature.
Despite the lack of neoangiogenesis, we recorded an increase in vascular permeability with leakage of plasma and uptake by neurons, similar to other studies [9, 16]. This leakage of BBB could be secondary to factors other than angiogenesis such as VEGF or AQP4 upregulation. Friedman considered the association between BBB dysfunction and seizures to be that of the classic ‘chicken and egg’ dilemma [17]. Early response to the exudation of serum albumin and altered extracellular milieu is mediated by astrocytes through transforming growth factor beta (TGF-β) signalling and phosphorylation of the Smad-2/5 pathway. Blocking the TGF-β pathway, following experimental BBB opening, has been shown to decrease the albumin-induced transcriptional response and prevent epileptogenesis. These animal models of status epilepticus have also demonstrated serum albumin and IgG within hippocampal neurons [18, 19]. The uptake of serum albumin is followed by down-regulation of the inward-rectifying potassium (K+) channels (Kir 4.1) on astrocytes, facilitating N-methyl-D-aspartate (NMDA) receptor-mediated neuronal hyperexcitability. This is further augmented by redistribution of AQP4 from the perivascular endfeet, contributing to impaired water flux and K+ buffering. This altered extracellular milieu could lead to uncontrolled neuronal firing [6].
VEGF is known to be upregulated in conditions of stress and hypoxia [20]. It could be either harmful or beneficial after seizures. In pilocarpine-induced status epilepticus animal models, an increase in VEGF protein in both neurons and glia has been noted. Increased VEGF contributes to BBB breakdown with reduced astroglial endfeet surrounding the vessel wall, along with leukocyte extravasation and inflammation. Nevertheless, experimental studies have also shown VEGF to be neuroprotective by shielding the vulnerable neurons from damage. However, the precise role of VEGF following seizure activity still needs to be elucidated [5, 21]. In line with other studies, we found increased VEGF expression in the resected hippocampi of HS cases relative to controls [9]. The upregulated VEGF may not necessarily drive the angiogenic pathway, but instead may be epileptogenic by disrupting the BBB or be neuroprotective to the neuron.
AQP4 is the most abundant water channel in the CNS with highest expression on perivascular astrocytic endfeet. Polarized expression of AQP4 is seen as maximum density close to vessels at the BBB. Loss of polarity of AQP4 denotes mislocalisation with diffuse distribution on the astrocytes [22]. Sclerotic hippocampi in medial temporal lobe epilepsy (mTLE) show an apparent elevated diffusion coefficient on diffusion-weighted imaging and T2 signal density on MRI suggesting accumulation of water [23]. Buffering of K+ depends on corresponding flux of water. Disturbances in K+ and water flux aggravate the potential for seizures. Hypo-osmolarity and decrease in extracellular space produces hyperexcitability. Also, increase in K+ concentration induces epileptiform activity. Restoration of water and K+ homeostasis by targeting AQP4 represents a novel therapeutic concept [24]. On immunohistochemistry, labelling of AQP4 is seen as a stippled perivascular patchy pattern in astrocytic processes around vessels in normal brain. We found a distinct difference in the pattern of labelling for AQP4 in HS cases compared to controls, with diffuse, intense labelling, suggesting redistribution or relocalisation from normal polarity in HS cases. However, this needs to be confirmed by ultrastructural studies. The marked increase in AQP4 labelling in cases is attributed to dense gliosis, as reported in other studies [25, 26].
Leakage of plasma, as labelled by IgG and albumin, did not show any correlation with vascular density, hippocampal volume, age at onset of seizures, duration of seizures or seizure frequency. Of the cases, 70% showed perivascular labelling for IgG and 87% for albumin, with neuronal and astrocytic uptake. The remaining cases revealed either complete absence or mild labelling of blood vessels without any exudation. This reinforces the fact that multiple aetiologies play a role in the aetiopathogenesis of HS, and that increased vascular permeability might be an epiphenomenon leading to perpetuation of seizures. The limitation of this study is that these findings were not compared to those of a similar cohort without HS, but rather epilepsy due to other aetiologies.
In short, we surmise from our observations based on the present study that there is no angiogenesis in HS, and the apparent increase in vasculature is relative to shrinkage/sclerosis of the hippocampus. However, we hypothesise that the increased permeability of the BBB caused by increased expression of VEGF or altered glia-vascular interface induced by modified localization of AQP4, and leakage of plasma followed by its uptake by neurons, could be a triggering factor for excitotoxicity, contributing to epileptogenesis.
This is the only study to date that has used a comprehensive approach towards understanding the pathobiology of HS by simultaneously evaluating and correlating several parameters such as angiogenesis, vascular density, hippocampal volume, BBB integrity, and the clinical characteristics in the same cohort.
Conclusion
The present study shows no objective histological evidence of angiogenesis in HS. No difference in vascular density was noted between HS and controls and no correlation was revealed between vascular density and hippocampal volume assessed by MRI, when controlling for the confounding variable of area (shrinkage of hippocampus due to neuronal loss and gliosis). Alteration of vascular permeability with uptake of serum IgG/albumin within neurons and astrocytes, along with redistribution of AQP4, contributes to epileptogenesis.
Supplementary material
Summary slides and supplementary table accompanying the manuscript and are available at www.epilepticdisorders.com.
Acknowledgements and disclosures
The authors acknowledge the Department of Science and Technology, Government of India for financial support vide Reference No. DST/SATYAM/2017/133, under the Science and Technology of Yoga and Meditation (SATYAM) to carry out this work.None of the authors have any conflicts of interest to disclose.