European Journal of Dermatology
MENUPathological nerve patterns in human basal cell carcinoma Volume 31, numéro 3, May-June 2021
Several reports have demonstrated the active role of nerve fibres in the tumour microenvironment of different cancers [1-5] through a direct exchange between tumours and nerve fibres [6]. Tumours influence the extracellular environment through neurotrophic mediators, while, peri-tumoral nerve fibres release neuro-transmitters in proper neuro-neoplastic synapses, enhancing tumour development and progression [7-11]. Basal cell carcinoma (BCC) is the most common of all cancers. The aim of this study was to perform a detailed investigation of the anatomical interactions between nerve fibres and BCCs, both in the tumour mass and in the peritumoral environment.
Materials and methods
A prospective observational study was carried out in cooperation with the Plastic Surgery Unit of the University of Pavia (Italy), the Pathological Anatomy Section Laboratory and the Neurophysiology Laboratory of the ICS Maugeri SB SpA IRCCS in Pavia (Italy). The study conformed to the 1975 Declaration of Helsinki; informed written consent was obtained from all of the patients and the trial was approved by the Ethics Committee of the Istituti Clinici Scientifici Maugeri SB SpA IRCCS, Pavia (Italy) (project identification code 2198).
The inclusion criteria were:
- –Patients aged 18 or over.
- –BCC sized 6 mm or more.
- –Any anatomical site.
The exclusion criteria were:
- –BCC sized less than 6 mm.
- –Recurrent and/or persistent BCC on a scar.
- –Unclear histological diagnosis.
- –Patients with multiple neoplasms and/or genetic diseases.
Over a period of six months, from October 2017 to March 2018, a total of 10 samples of histologically diagnosed BCC, derived from 10 different patients (five females and five males; age: mean 64.2, median 74), was included in the study.
Surgical excisions of clinically diagnosed BCCs were carried out with a 5-mm margin in healthy tissue according to the Italian Association of Medical Oncology (AIOM) 2017 guidelines. Two 3-mm punch biopsies, including the skin and the subcutaneous layers, were harvested for each surgical specimen. One 3-mm punch biopsy was harvested within the tumour mass, while the other was harvested in the clinically healthy skin around the tumour. The punch biopsies never extended to either the superficial or the deep margins of the surgical specimen, thus preventing any bias in the oncological diagnosis by the pathologist.
Haematoxylin and eosin staining
The surgical specimens were fixed with 10% neutral buffered formalin (pH 7.2) (Bio-Optica, Milan, Italy) for 24 hours at room temperature, and were then dehydrated through graded concentrations of ethanol (ASP300S Leica Microsystems Srl, Buccinasco, Milan, Italy) and embedded in paraffin. Subsequently, 3-μm-thick sections were obtained with an automatic microtome (HM355S Thermo Fisher Scientific, Waltham, MA, USA), rehydrated and subsequently processed with Harry's Hematoxylin (Bio-Optica) for 4 minutes and Eosin (Bio-Optica) for 4 minutes at room temperature (ST5020 and CV5030 Leica Microsystems).
Immunofluorescence staining
The punch biopsies were fixed with Zamboni solution (2% paraformaldehyde and picric acid) at 4 °C for 24 hours and subsequently cryoprotected in a 20% sucrose solution with a 7.4 pH saline phosphate buffer. The samples were then placed in Optimum Cutting Temperature (OCT) gel and sliced in 50-μ sections using a microtome provided with a refrigerating unit, working at -19 °C (HMG 450, Microm International, Walldorf, Germany).
Nerve fibres were stained for indirect immunofluorescence using primary rabbit polyclonal antibodies targeting protein gene product 9.5 (PGP 9.5). A secondary, species-specific antibody targeting the primary antibody was linked with cyanine 3 (Cy3), a fluorochrome of the cyanine family. Cyanine 3 displays green light with a maximum absorbance at 550 nm and a maximum light emission at 570 nm (yellow-orange). The dermal-epidermal junction was stained with a primary, mouse monoclonal antibody, targeting collagen IV fibres that are specific to the basal membrane. The species-specific secondary antibody targeting the primary antibody was linked to cyanine 2 (Cy2) fluorochrome which displays blue light, with maximum absorbance at 498 nm and maximum light emission at 505 nm (green) [12].
The sections were observed with a fluorescence microscope (Axioskop 40 FL, Zeiss, Gottingen, Germany) equipped with a digital camera (Axiocam MRC5, Zeiss) and dedicated image processing software (Axiovision Rel 4.8).
Results
In our sample, haematoxylin and eosin staining demonstrated seven different histological sub-types of BCC: four were classified as histologically aggressive according to Sexton [13] (one morphea-like, one basosquamous, one micronodular, two mixed nodular-micronodular) and three were histologically non-aggressive (two adenoid, two nodular, one superficial multifocal).
Overall, 247 stained micro-sections were analysed under the fluorescence microscope.
The immunofluorescence staining demonstrated the complete absence of nerve fibres within the tumour parenchyma in all of the samples. The nerve fibres were evident only within the stromal collagen.
Three different morphological patterns of nerve fibre distribution were identified at the interface between the tumour parenchyma and stromal collagen. A number of patterns coexisted in the same section in all of the samples (minimum of two, maximum of three).
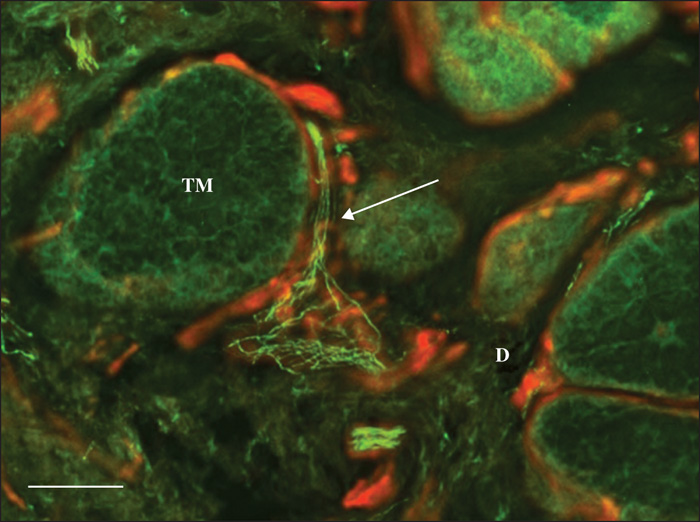
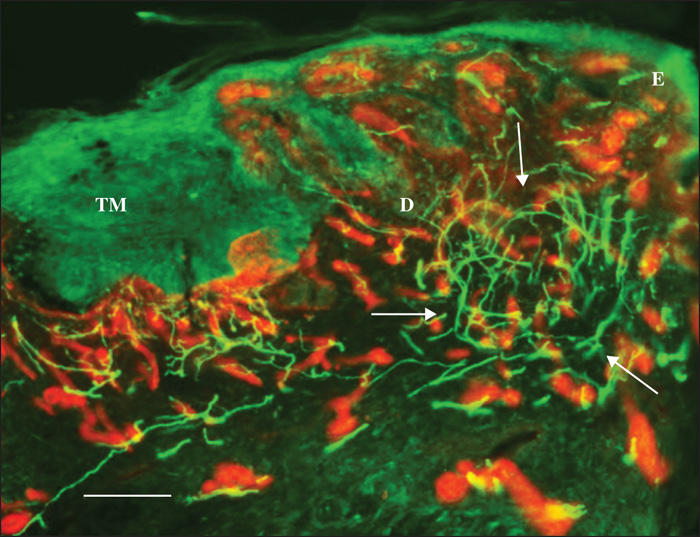
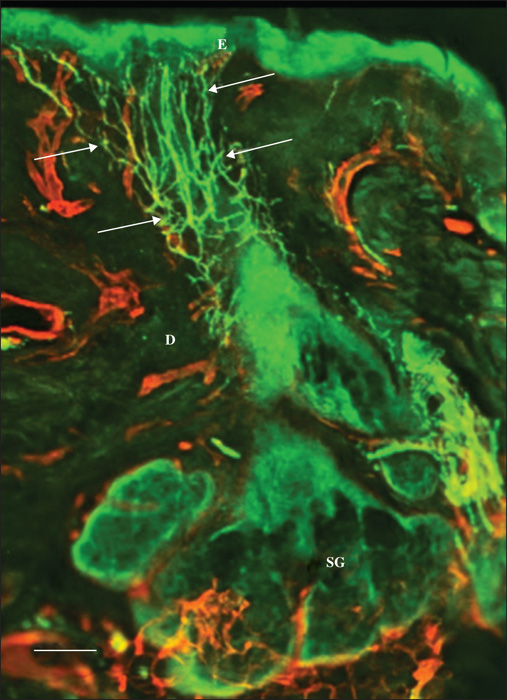
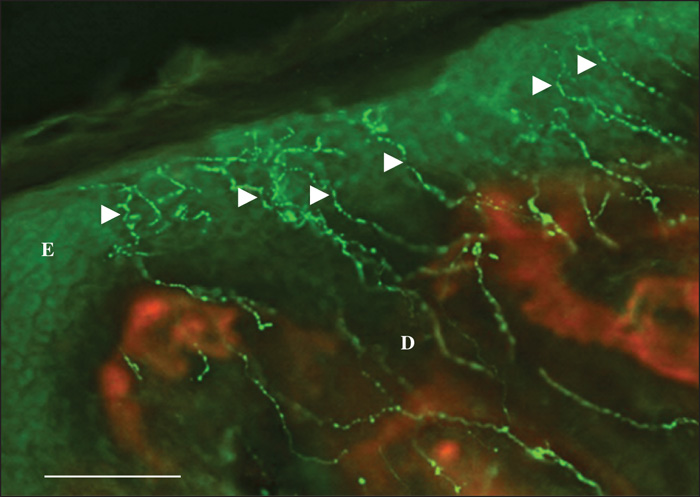
Pattern 1 displayed normal nerve fibres that were dislodged by the tumour masses. Occasionally, stromal septa, including nerve fibres, were found to divide the tumour masses into multiple lobes (figure 1). Pattern 2 featured a chaotic aggregation of thin curved and tangled nerve fibres in the peritumoral stroma (Figures 2, 3). There was no correlation between Patterns 1 and 2 and any of the histological types. Pattern 3 was demonstrated only in the nodular BCCs, and featured a localized crowding of nerve fibres running between the tumour and the overlying epidermis. The fibres penetrated into the epidermis and focal epidermal hyperinnervation was appreciated. The nerve fibres also displayed some focal swelling that might be related to axon degeneration (figure 4).
Discussion
The onset of BCCs has been demonstrated to occur preferentially at sites of congenital craniofacial and neck clefts, which, in adulthood likely correspond to the sites of fusion and/or merging of embryonic processes [14-17]. Similarly, anomalies in the Hedgehog (Hh) signalling pathway play a major role in craniofacial congenital malformations [18, 19], from survival of migrating cranial neural crest cells [20] to fusion of craniofacial prominences [21-26].
Dysregulation of the Hh signalling pathway has been demonstrated in human BCC [27-30]. During both development and homeostasis, the Hh signalling pathway is finely tuned by a balance of upstream factors that can either promote its activation, such as Smoothened (SMO), or suppress it, such as Patched1 (PTCH1). In BCC, this balance is markedly tilted in favour of pathway activation through mutations yielding either loss of PTCH1 function, or, the stable activation of SMO [31, 32]. Evidence of a perturbed Hh signalling pathway in human BCC was indicated in studies demonstrating that patients with defective PTCH1 alleles are predisposed to developing multiple BCCs (Gorlin's syndrome) [31, 33, 34].
Recently, Peterson et al. (2015) [35] demonstrated in PTCH1-deleted mice, treated with tamoxifen, that BCC was more likely to develop from specific cell clusters, defined as “hot spots”. More specifically, neoplastic degeneration was demonstrated in upper and lower bulge stem cells of the hair follicle, and in small inter-follicular cell clusters close to highly innervated zones called “touch domes” (TDs).
Within the hair follicle, the nerve fibres form the so-called “piloneural collar”. A sort of delicate basket, it includes a variety of sensory afferents and terminal Schwann cells that innervate a region below the sebaceous gland, but above the hair follicle upper bulge [36]. The piloneural collar is typically structured in an inner layer of nerve fibres which run in a longitudinal direction forming palisades of lanceolate nerve endings, and an outer layer of fibres with a circumferential pattern.
The TDs are specialized structures in the papillary dermis of hair-bearing skin that approach the hair follicle without penetrating it. They are made of specialized Merkel cells that detect and transmit light and touch sensations through the underlying slowly adapting type 1 sensory afferent nerve fibres [37, 38]. In the TDs, the nerve fibres approach the epidermis, branching into smaller fibres that crowd in the sub-epidermal region, eventually penetrating the epidermis to join Merkel cells [39-41].
According to Peterson (2015) [35], direct involvement of the nervous system in the development of BCC, as well as in other malignancies [4], might be suggested, as the skin “hot spots” display a tight interconnection with the nervous tissue. Their study demonstrated that the skin-sensitive nerve fibres express the Hedgehog ligand, and that the skin denervation interferes with Hh activity in the TDs, blunting tumour development and progression from these cells. These results suggest that the skin nerve endings produce the Hh ligand that, in turn, triggers tumour development at the TD site. Nevertheless, as TD-derived tumours do not respond to anti-Hh ligand antibodies, the involvement of multiple different types of Hh ligands might be hypothesized in the process of cancer development. Thus, in order to prevent the Hh-ligand carcinogenic effect, it would seem appropriate to deactivate the Hh receptor with permanent denervation [35]. Other studies, meanwhile, have demonstrated that the Hh pathway might also be activated by TGF-beta [42].
Our observations demonstrate a total of three morphological nerve patterns within BCC samples. Pattern 1 did not show actual pathological features, as it displayed a normal skin nerve pattern in which the fibres were simply dislodged and splayed by the growing tumour masses. Patterns 2 and 3, on the other hand, displayed a clear pathological trait: Pattern 2 featured a ball of curved, tangled nerve fibres close to the tumour masses resembling the piloneural collar nerve fibres that wrap around the hair follicle in the normal anatomical setting. Pattern 3, meanwhile, showed nerve fibre crowding in the sub-epidermal layer with the fibres approaching the epidermis. Such a pattern might call to mind the typical anatomical neuro-epithelial interaction in a TD.
Our study failed to demonstrate a correlation between the different nerve distribution patterns and the histological types of BCCs and their clinical variations. Undoubtedly, a larger sample could potentially demonstrate more reliable results. However, the comparative complexity of the coordinated clinical and experimental setting, we believe, justifies the small-sized sample in our trial.
The epidermis and skin adnexa share the same ectodermal origin as nerve tissue, and in adult skin they demonstrate a close mutual anatomical and functional interaction.
The peculiar combined, or binary, involvement of epithelium and stroma makes BCC a unique tumour versus other epithelial malignancies, in which individual epithelial cells transform into cancer cells that subsequently feature a stroma-dissociated invasiveness [43]. On the other hand, skin adnexal primordial tissue with combined fibro-epithelial growth is always retained in BCC. Such a unique pattern supports the alternative concept of a monstrous attempt at skin adnexogenesis in BCC in adults, rather than proper malignancy [44]. Therefore, considering the common neuro-ectodermal origin of both the epidermis and nerves, a mutual active interaction between these tissues might be expected in BCC.
Our study may have disclosed a hidden third player, of nerves, thus, rather than binary tissue interaction in BCC, the following triad may be involved: the epithelium, stroma and nerves, with each component potentially retaining some features associated with its developmental setting. The protective effect resulting from selective denervation, based on BCC in experimental animals, might be explained by suppression of one of the tissues actively involved in mutually interactive development and proliferation. Therefore, in the specific case of BCC, nerves might be one element in the context of combined organoid proliferation, rather than direct carcinogenetic inducers [35].
Such evidence may provide further support to the hypothesis of embryological pathogenesis of BCC, and perhaps lead to suggestions for novel preventive and therapeutic proposals.
Acknowledgements and disclosures
Acknowledgements: The Authors wish to thank Morag McGhee (MA) and Eleanor Susan Lim (MA, Hons) for their contribution to the submission of this dissertation. Financial support: none. Conflicts of interest: none.
* The study was carried out in Pavia (PV), Italy.