Epileptic Disorders
MENUEffectiveness and safety of perampanel in Chinese paediatric patients (2-14 years) with refractory epilepsy: a retrospective, observational study Volume 23, numéro 6, December 2021
- Mots-clés : levetiracetam, oxcarbazepine, paediatric patients, perampanel, refractory epilepsy
- DOI : 10.1684/epd.2021.1342
- Page(s) : 854-64
- Année de parution : 2021
Objective
We report our clinical experience of the effectiveness and tolerability of adjunctive perampanel treatment in a Chinese paediatric population with refractory epilepsy. We also compare the effectiveness and tolerability of perampanel with or without concomitant oxcarbazepine or levetiracetam.
Methods
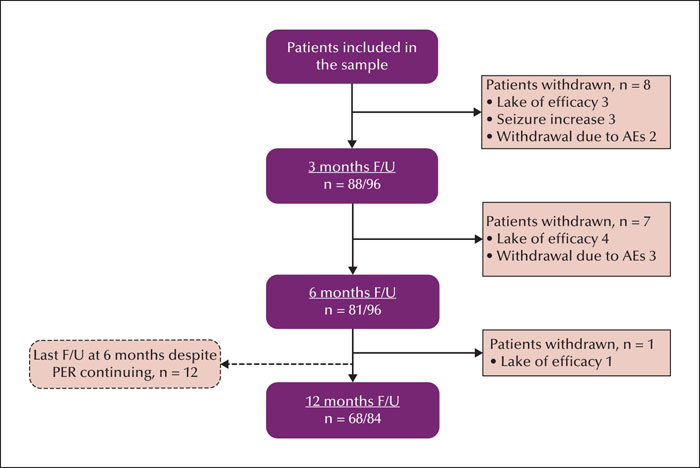
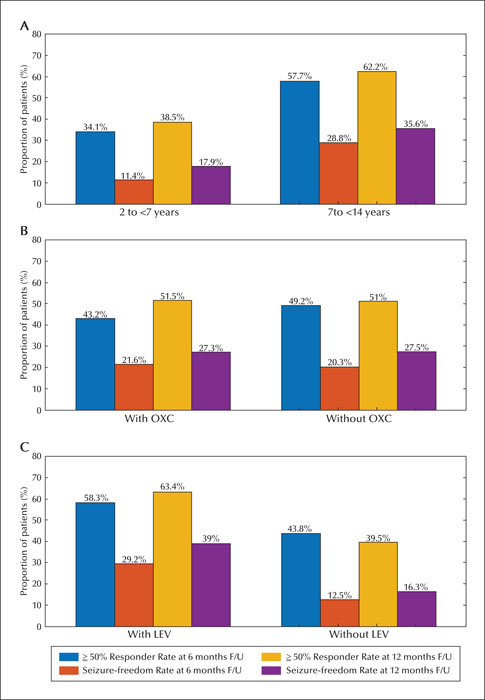
This retrospective observational study was conducted from September 2019 to September 2020 in the paediatric neurology clinics of two tertiary hospitals in the Chinese mainland. We reviewed the data obtained from 96 paediatric patients aged 2-14 years whose seizures were pharmacoresistant and who could be followed up for a minimum of six months. The effectiveness was estimated by the perampanel response rate at 6- and 12-month follow-up evaluations. Adverse events were also recorded. Patients were stratified by age (2-7 and 7-14 years), and with/without concomitant oxcarbazepine or levetiracetam.
Results
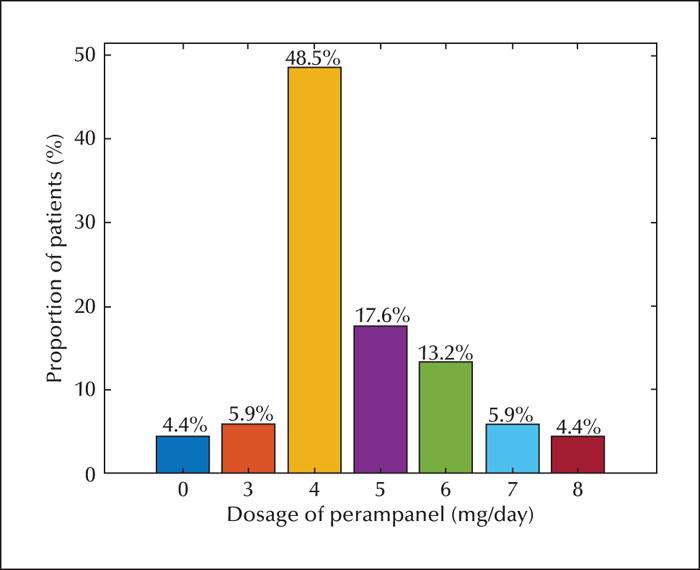
The population comprised 96 patients with refractory epilepsy. The retention rate was 84.4% and 81.0% at 6 and 12 months, respectively. The most common dose used was 4 mg (48.5%). Corresponding 50% responder and seizure freedom rates were as follows: 46.9% and 20.8% at six months, and 51.2% and 27.4% at 12 months, respectively. Adverse effects were reported in 22 patients (22.9%). The most common adverse effects were irritability, somnolence, and dizziness. The 50% and 100% responder rates and adverse effects were greater in patients aged 7-14 years than in those aged 2-7 years. The proportion of responder and seizure-free rates and adverse effects were similar with or without oxcarbazepine. Perampanel was more effective in patients concomitantly treated with levetiracetam, however, this did not result in significantly more adverse events, including aggression.
Significance
The present study suggests that perampanel, with or without concomitant oxcarbazepine or levetiracetam, is generally safe, well-tolerated, and efficacious in paediatric patients with uncontrolled epilepsy in a clinical setting.