Epileptic Disorders
MENUCutaneous adverse reactions associated with antiseizure medication: clinical characteristics and implications in epilepsy treatment Volume 23, numéro 3, June 2021
- Mots-clés : antiseizure medication, cutaneous adverse reaction, cross-sensitivity, epilepsy, antiepileptic
- DOI : 10.1684/epd.2021.1288
- Page(s) : 466-75
- Année de parution : 2021
Objective. To describe the clinical characteristics of cutaneous adverse reactions and cross-sensitivity induced by antiseizure medications and compare the pattern of use of antiseizure medications in patients with epilepsy according to skin rash history.
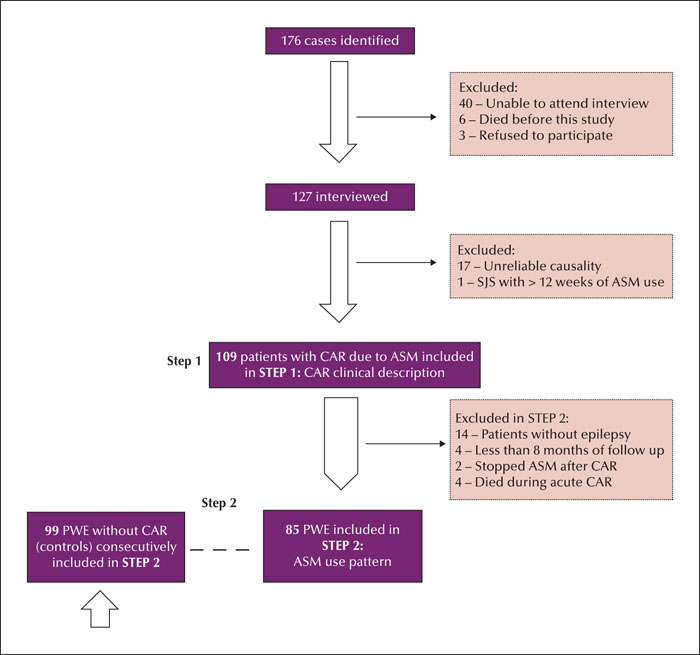
Methods. We analysed patients with a history of skin rash presenting for up to 12 weeks after initiating antiseizure medication. The history of skin rash was verified by medical charts, interviews, and identification of skin lesions by patients based on illustrative images. The minimum follow-up period was eight months. The control group comprised epilepsy patients with regular antiseizure medication use for at least 12 weeks without skin rash. We included 109 cases and 99 controls.
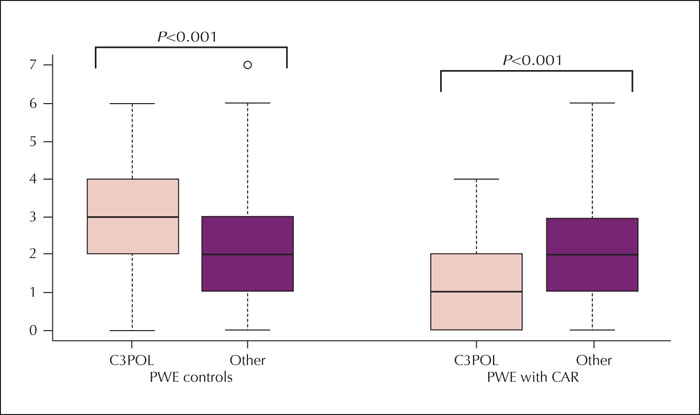
Results. The median (interquartile range) period from the index rash was six years (2-11). Carbamazepine was the trigger medication in 48% of cases and induced skin rashes in all patients with cross-sensitivity and carbamazepine exposure. Stevens-Johnson syndrome, toxic epidermal necrolysis, or drug reactions with eosinophilia and systemic symptoms affected 36% of cases. Carbamazepine- or oxcarbazepine-induced maculopapular exanthema occurred earlier (median: one week) than that induced by other antiseizure medications (median: three weeks) (p=0.006). Cross-sensitivity was more common in patients with at least one episode of Stevens-Johnson syndrome (29%) and Stevens-Johnson/toxic epidermal necrolysis overlap (50%) than in patients with maculopapular exanthema (8%) (p=0.01). Although most cases were mild, the pattern of antiseizure medication use differed from that of controls, with a lower proportion of antiseizure medication typically associated with severe cutaneous adverse reactions (carbamazepine, phenytoin, phenobarbital, primidone, oxcarbazepine, and lamotrigine) (p<0.001). Most cases exposed to high-risk medication, however, did not develop cross-sensitivity.
Significance. Cutaneous adverse reaction history may influence antiseizure medication use. Cross-sensitivity is more common in severe cases and most patients are affected by mild, self-limited skin rashes. Further research should consider the relevance of mild skin rashes in lifelong epilepsy treatment.