European Journal of Dermatology
MENUEfficacy and safety of nivolumab in metastatic melanoma: real-world practice Volume 29, numéro 3, May-June 2019
- Mots-clés : advanced melanoma, anti-PD1, real-life study
- DOI : 10.1684/ejd.2019.3558
- Page(s) : 315-21
- Année de parution : 2019
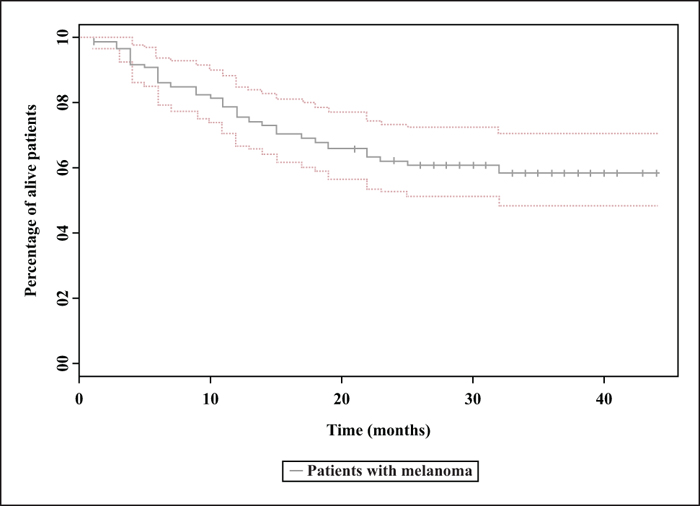
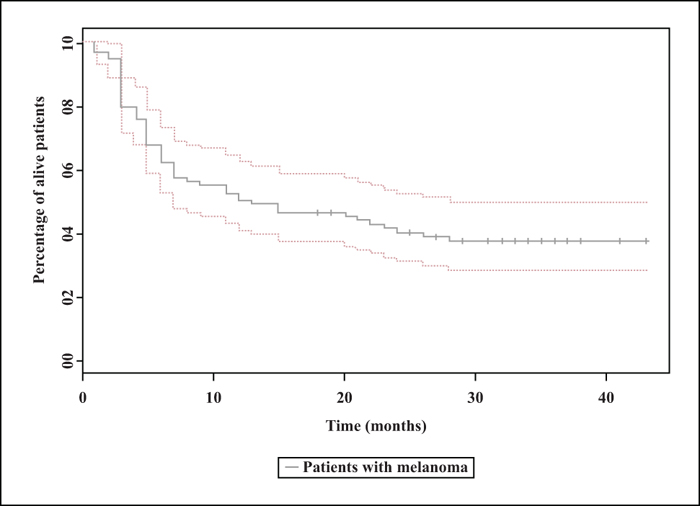
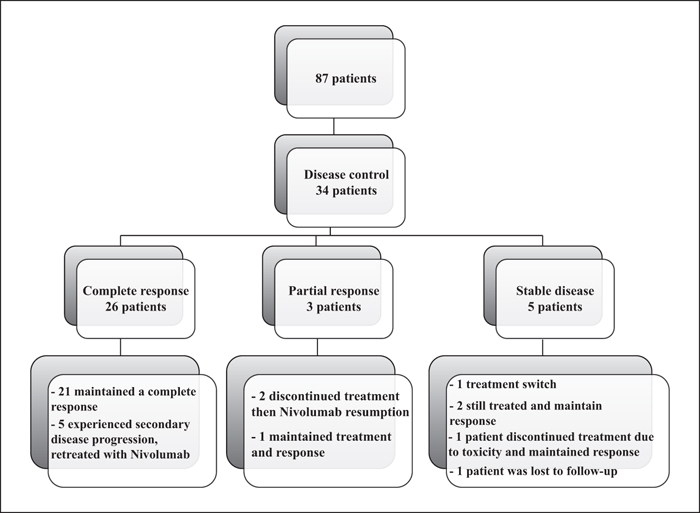
Background: Anti-PD1 antibodies have revolutionized the management of patients with advanced melanoma. In clinical trials, the efficacy of nivolumab is being tested in selected populations of patients. Objectives: The aim of this study was to analyse the efficacy and safety of nivolumab in patients with advanced melanoma under real-life conditions. Materials and Methods: A retrospective, observational study was conducted in patients treated with nivolumab for advanced melanoma included in the RIC-Mel network. Overall survival and progression-free survival (PFS) were assessed using the Kaplan-Meier method. Results: Eighty-seven patients were included with a median follow-up of 31 months. The median PFS was 13 months (95% CI: 7-28). Objective response rate was 33.3%. Among patients achieving a complete response, the response was maintained after treatment discontinuation in 80.7% of patients for a median duration of 21.7 months. Multivariate analysis showed that an increased lactate dehydrogenase level (p = 0.03; HR: 1.21; 95% CI: 1.02-1.45) and brain metastases (p = 0.024; HR: 2.78; 95% CI: 1.14-6.77) were correlated with a decrease in PFS. Grade 3 or 4 adverse events were found in 10.3% of patients. Conclusion: Based on our study, the efficacy and safety of nivolumab in patients with advanced melanoma are consistent with previously published data.