European Journal of Dermatology
MENUAdapalene/benzoyl peroxide gel 0.3%/2.5% for acne vulgaris Volume 32, numéro 4, 2022-07-01
- Mots-clés : acne vulgaris, atrophic acne scars, scarring, adapalene, benzoyl peroxide, fixed-dose combination, severe acne
- DOI : 10.1684/ejd.2022.4275
- Page(s) : 445-50
- Année de parution : 2022
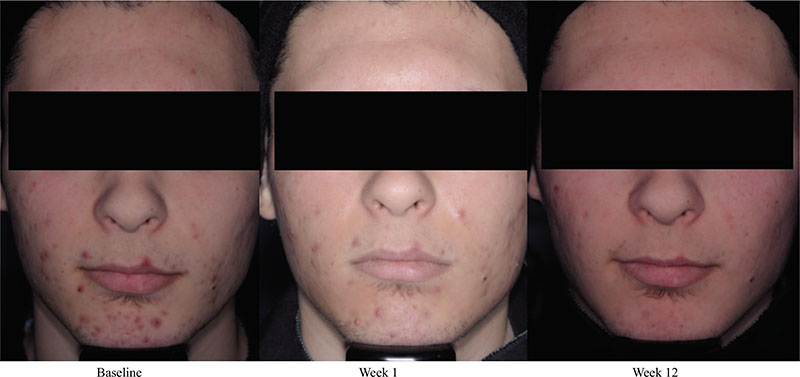
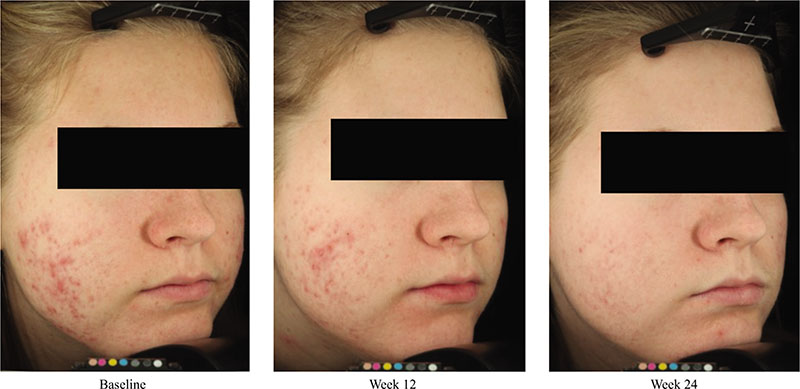
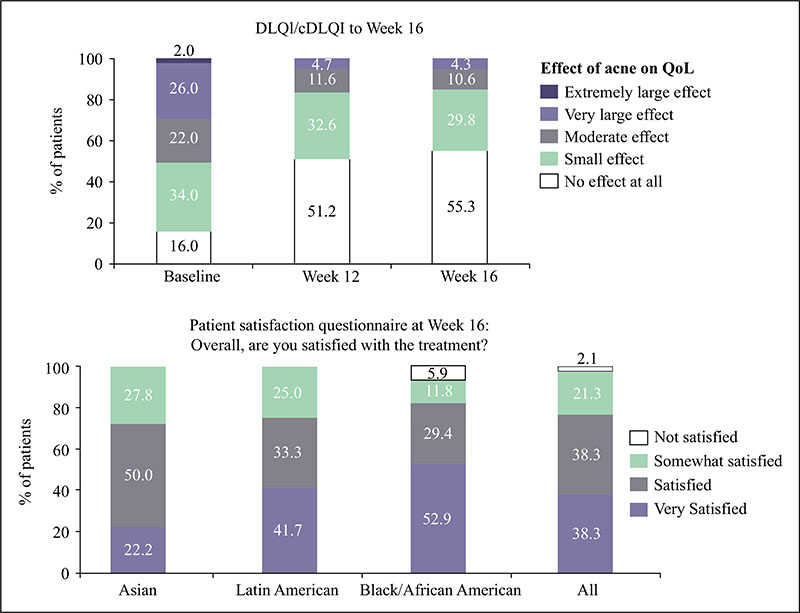
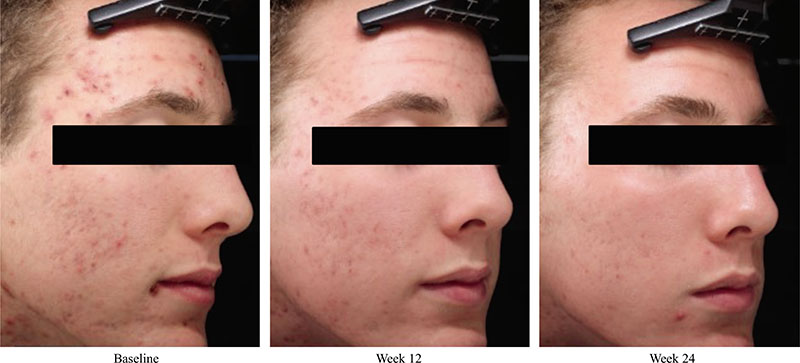
Acne vulgaris is typically treated with a combination of a topical retinoid plus an antimicrobial agent, as recommended by national and international evidence-based guidelines around the globe. Adapalene, a synthetic topical retinoid, is available in two concentrations (0.1% and 0.3%) and in once-daily fixed-dose combinations with benzoyl peroxide (BPO) 2.5%. Adapalene 0.3%/BPO 2.5% is approved for use for moderate-to-severe acne with proven efficacy, good safety and tolerability across a spectrum of patient variables (different ages, genders, and skin types) and disease severity. While some patients experience issues with transient tolerability during retinoid and BPO therapy, it is our clinical experience that good patient education to set expectations and provide strategies to minimize irritation can overcome the majority of issues. This article reviews the data supporting the use of adapalene 0.3%/2.5% in practice, including the complementary mechanism of action of adapalene and BPO, clinical data from a range of settings, and key aspects of patient education.