European Journal of Dermatology
MENUPredictive factors of response to vismodegib: a French study of 61 patients with multiple or locally advanced basal cell carcinoma Volume 32, numéro 3, May-June 2022
- Mots-clés : basal cell carcinoma, Gorlin syndrome, hedgehog pathway inhibitor, long-term, vismodegib
- DOI : 10.1684/ejd.2022.4264
- Page(s) : 401-7
- Année de parution : 2022
Background
Vismodegib is indicated for the treatment of advanced or metastatic basal cell carcinoma (BCC). The predictive factors of response to vismodegib have so far been poorly described.
Objectives
The primary objective was to determine the profile of patients responding to vismodegib and the duration of response. Secondary objectives were to assess whether there is a correlation between the duration of treatment and the risk of relapse, and to define factors associated with relapse.
Materials & Methods
We included 61 patients with locally advanced BCC (laBCC) or multiple BCC, treated with vismodegib (150 mg per day), from July 2011 to November 2015, in the Oncodermatology Department of Nantes University Hospital in France. Tumour response was assessed using Response Evaluation Criteria in Solid Tumours version 1.1.
Results
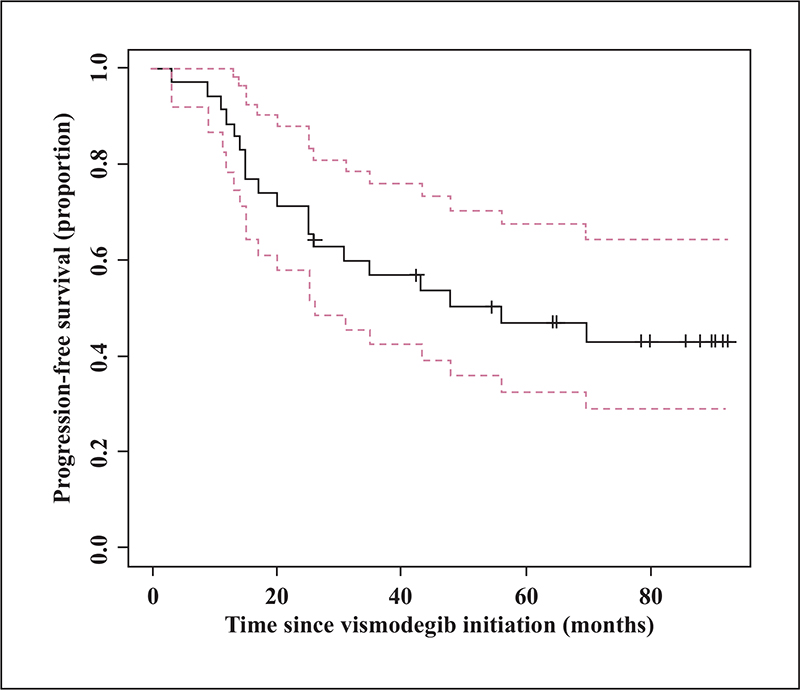
Thirty-nine patients had advanced BCC (64%) and 22 patients multiple BCC (36%), including 10 patients with Gorlin syndrome. No factor predicted response to vismodegib. The median progression-free survival (PFS) was 69.5 months for the total population. In multivariate analysis, multiple BCC was the only factor associated with an increased risk of relapse (HR: 13.80 [CI95%, 1.93-98.64, p < 0.01]). Treatment duration decreased the risk of relapse (HR 0.95 [CI95%, 0.90-0.99, p = 0.0467]). Among the 20 patients who experienced relapse during follow-up, 15 (75%) were re-treated with vismodegib, with a response rate of 66%.
Conclusion
Although we were unable to establish predictive factors for the response to vismodegib, we demonstrate for the first time that increased treatment duration correlates with a decreased risk of relapse.