Epileptic Disorders
MENUMowat-Wilson syndrome presenting with fever-associated seizures Volume 19, numéro 4, December 2017
Mowat-Wilson syndrome (MWS) is a disorder caused by mutations or deletions of the ZEB2 gene. It is characterized by distinctive facial features, cognitive impairment, and multiple anomalies, including cardiac, genito-urinary, and eye defects (Mowat et al., 1998). Seizures are also a common manifestation, occurring in 70% of patients. However, MWS often goes unrecognized and undiagnosed by neurologists, because the seizures vary and underlying genetic abnormalities may be overlooked using conventional genetic tests (Buraniqi and Moodley, 2015).
Fever-related seizures represent the most common seizure type in children. It can be challenging to differentiate between patients who will have only febrile seizures in their lifetime from those who will develop other seizure types in the future. The latter includes severe epilepsy syndromes, such as Dravet syndrome and protocadherin 19 (PCDH19)-related epilepsy.
Here, we describe two patients with MWS who presented with atypical forms of fever-related seizures that were different from previous typical cases. We were able to genetically confirm diagnosis of these patients using a targeted next-generation sequencing (NGS) gene panel for epilepsy.
Materials and methods
For the customized NGS gene panel, we selected 172 candidate genes that are known to cause epilepsy. Genomic DNA was extracted from leukocytes of whole-blood samples. Pooled libraries were sequenced using a MiSeq sequencer (Illumina, San Diego, CA, USA) and the MiSeq Reagent Kit v2 (300 cycles).
Case 1
A 4-year-old girl came to our outpatient clinic due to fever-triggered seizures. She was delivered vaginally at full term, with a birth weight of 3,300 g without any postnatal complications. Her seizures began when she was 10 months old and occurred yearly thereafter.
Seizures were generalized, of tonic-clonic type, lasted 10 minutes, and always occurred with febrile illnesses. At 4 years of age, her seizures changed into a generalized tonic type and occurred in clusters. Each seizure lasted about 10 minutes and occurred five to six times per day in a row. Nonetheless, seizures continued to occur only with febrile illnesses. Her parents did not report any focal features of the seizures.
Other than seizures, the patient had two left ureters and hydronephrosis, and profound developmental delay. At the age of 5 years, she could barely walk and could not talk. There was no family history of any neurological or developmental disorders, or other congenital malformations.
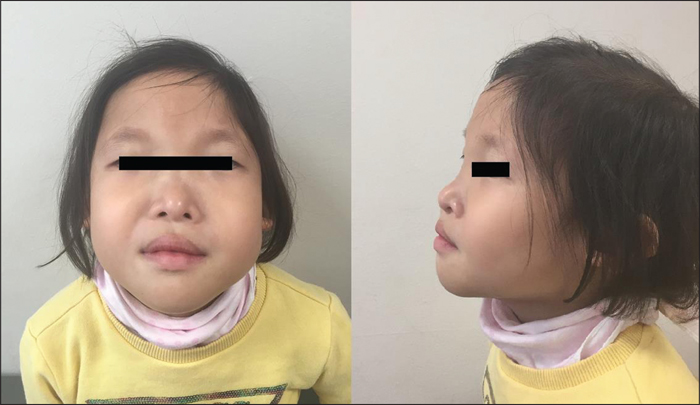
A physical examination revealed facial dysmorphisms, characterized by large eyebrows with medial flaring, hypertelorism, telecanthus, a broad nasal bridge, a prominent chin, and a prominent columella (figure 1). She was short in stature, with a height of 95 cm (6th percentile) and a body weight of 15 kg (31st percentile). A neurological examination did not show any other abnormalities.
Brain MRI and EEG did not reveal any abnormalities. Performed genetic tests included chromosomal analysis, SCN1A gene screening, and array CGH, which were all negative. We applied a targeted NGS gene panel for epilepsy and identified a nonsense mutation in the ZEB2 gene in which a TAC codon was mutated to a TAA stop codon (c.1965C>A [p.(Tyr652*)]) (figure 2). She was subsequently diagnosed with MWS. Valproic acid therapy was initiated, and at the current age of 5 years and 3 months, she has been seizure-free for 14 months.
Case 2
A 24-month-old boy was admitted to our hospital due to a fever-triggered seizure. He was born by Caesarean section at full term, with a birth weight of 3,710 g without any postnatal complications. He first had a fever-triggered seizure when he was 18 months old. His seizure was a right hemiconvulsive seizure that lasted about 10 minutes. After five months, he had another fever-triggered seizure, a left hemiconvulsive seizure. This seizure also lasted for 10 minutes. At 2 years of age, he began to have afebrile seizures every two or three months. These seizures started with right head deviation with or without secondary generalization. These seizures lasted for 10 minutes each.
His development was significantly delayed and he could only walk, not run, at the age of 36 months. He could speak only two words. He had right cryptorchidism, bilateral hydrocele, a concealed penis, and penile torsion, which required surgery. A physical examination revealed facial dysmorphisms, characterized by eyebrows with medial flaring, hypertelorism, telecanthus, a broad nasal bridge, and a prominent columella. He was short in stature, with a height of 77.2 cm (7th percentile), although his body weight was 10.8 kg (34th percentile). A neurological examination did not show any other abnormalities.
Brain MRI did not reveal any abnormalities. EEG performed at the age of 30 months showed frequent multifocal epileptiform discharges from the left or right parietal and temporal areas. Multiple ligation-dependent probe amplification (MLPA) and SCN1A genetic testing were performed; all test results were negative. We performed targeted NGS for epilepsy and identified a frameshift mutation in the ZEB2 gene (c.2348dupC [p.Ser784PhefsTer11]). He was subsequently diagnosed with MWS. Valproic acid therapy was initiated at 25 months of age, although recurrent afebrile seizures continued to occur every two months. Because of thrombocytopenia, his medication was changed to levetiracetam. Presently, he has a brief focal afebrile seizure every three or four months.
Discussion
Diagnosing patients with fever-induced seizures can be challenging. Our cases suggest that MWS can initially present with fever-induced seizures. Although rare, MWS should be considered in the differential diagnosis for fever-induced seizures, especially when the patient has facial dysmorphisms, congenital anomalies, and developmental delay altogether.
Our two patients with MWS presented with atypical forms of fever-triggered seizures. One patient had clusters of six to seven prolonged seizures per day, accompanied by fever. Another patient had prolonged hemiconvulsions with febrile illnesses. The prolonged duration, clustered nature, and focality of the seizures resembled fever-triggered seizures seen in patients with Dravet syndrome, PCDH19-related epilepsy, or structural epilepsy. Many MWS patients are known to present with fever-triggered seizures before they develop afebrile seizures (Cordelli et al., 2013). However, the characteristics of these fever-triggered seizures have not been addressed specifically. Our study suggests that fever-triggered seizures in MWS may have different features that distinguish them from other “simple” febrile seizures. Since EEG and MRI results may be normal, the role of these techniques may remain limited in the diagnosis of MWS (Cordelli et al., 2013). Identifying characteristic initial seizure types of MWS would appear to be very important.
Valproic acid seems to be the most commonly used, effective, antiepileptic drug to treat patients with MWS (Cordelli et al., 2013). Clusters of seizures were resolved with valproic acid therapy in one of our patients. The impact of early intervention with appropriate drugs must be assessed in future studies. Overall, 70% of patients with MWS develop seizures, and more than 50% of MWS patients develop drug-resistant epilepsy (Cordelli et al., 2013). Risk factors for drug-resistant epilepsy in MWS remain unknown.
MWS is a single-gene disorder that was first described by Mowat et al. in 1998 (Mowat et al., 1998). MWS is believed to be under-diagnosed because of its diverse manifestations and the broad spectrum of genetic mutations involved (Park et al., 2013; Babkina et al., 2016). Hirschsprung disease, congenital heart defects, urogenital/renal anomalies, and short stature occur, either as separate or multiple manifestations. Facial abnormalities are characteristic, although facial features can change with age (Garavelli et al., 2009). In early childhood, non-neurologists often see patients with MWS to address their heart or genital anomalies, and patients with MWS are often referred to neurologists after developing afebrile seizures during late childhood. A detailed history of febrile seizures and other medical conditions are critical in MWS, but often remain under-recognized. The clinical features of published cases of Mowat-Wilson syndrome with confirmed ZEB2 mutations, as well as the two patients described here, are presented in table 1.
Mutation in the ZEB2 gene is a known cause of MWS. Sequence analysis has been used to detect mutations in approximately 79% of affected individuals, while either quantitative PCR or multiplex ligation-dependent probe amplification (MLPA) have been used to detect intermediate-sized deletions in an additional 6% of subjects (Garavelli and Mainardi, 2007). Cytogenetic analysis is also recommended to exclude large deletions or translocations. It has been possible to detect submicroscopic deletions using FISH analysis in 13% of affected individuals. Nonsense mutations are common, but frameshift mutations caused by small deletions, duplications, and insertions are also not rare. Most mutations in the ZEB2 gene are identified by conventional genetic testing, however, due to the diversity of genetic abnormalities, an NGS epilepsy gene panel may be an option when clinicians have difficulties in diagnosing patients who present atypical features of MWS.
This study emphasizes that MWS should be considered in patients who present with fever-induced seizures in an atypical manner. When patients with delayed development and distinctive facial features develop intriguing fever-induced seizures, MWS can be suspected. Further genetic testing may be helpful for diagnosis. Larger studies are recommended for a more detailed characterization of fever-induced seizures in MWS.
Supplementary data
Summary didactic slides are available on the www.epilepticdisorders.com website.
Acknowledgements and disclosures
This research was performed at the Center for Pediatric Neurology, Severance Children's Hospital, Yonsei University College of Medicine, Seoul, Korea, and was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C1601).