Epileptic Disorders
MENUIctal asystole in temporal lobe epilepsy before and after pacemaker implantation Volume 10, numéro 1, march 2008
Auteur(s) : Adam Strzelczyk, Sebastian Bauer, Susanne Knake, Wolfgang H Oertel, Hajo M Hamer, Felix Rosenow
Department of Neurology, Philipps-University, Marburg,
Germany
Article reçu le 10 Octobre 2007, accepté le 26 Decembre 2007
Ictal bradycardia and asystole are autonomic symptoms that are rare during epileptic seizures, but they may contribute to sudden, unexplained death in epilepsy (SUDEP) (Baumgartner et al. 2001). SUDEP is probably the most important category of epilepsy-related deaths, and results in 2% to 18% of all deaths in patients with epilepsy (Pedley and Hauser 2002). However, despite growing interest in SUDEP, the underlying pathophysiology remains unclear. The criteria for defining SUDEP include patients suffering from epilepsy who die, unexpectedly and suddenly, in benign circumstances, while in a reasonable state of health, and without any obvious cause other than cardiac arrhythmia, while excluding status epilepticus and seizure-induced trauma and drowning (Leestma et al. 1997). The average incidence of SUDEP in patients with refractory epilepsy was calculated to be 3.7/1 000 patient-years (Ryvlin and Kahane 2003). An early onset of epilepsy, long disease duration, high seizure frequency and anti-epileptic polytherapy seem to be risk factors for SUDEP (Tellez-Zenteno et al. 2005, Tomson et al. 2005). However, up to now, no study has shown a direct link between ictal asystole and SUDEP. The few published cases of SUDEP with EEG recordings show a sudden flattening of the EEG prior to death (Bird et al. 1997, Lee 1998, McLean and Wimalaratna 2007). The authors suggest that the cause of death might be cerebrogenic and not cardiogenic, however, only pulse or electrocardiogram (ECG) artefacts were available, as a simultaneous ECG was not recorded in any of the three cases.
In a prospective, long-term ECG study of 20 patients with refractory partial epilepsies, a substantial number of the patients showed cardiac arrythmias. Events of ictal bradycardia or asystole were recorded in more than one third of the patients, resulting in subsequent permanent pacemaker insertion in four of them (Rugg-Gunn et al. 2004).
Leung et al. (Leung et al. 2006) summarised the scarce EEG data available on seizure localisation in ictal bradycardia and asystole. Electrical brain stimulation studies show that variation in heart rate, mostly bradycardia, can be induced by stimulation of the anterior cingulated cortex bilaterally, right orbitofrontal, right and left insular cortex as well as the left basotemporal region and the right uncus. Intracranial EEG data were sufficient in four patients but they did not show a uniform pattern on EEG. The EEG seizure in the right and left temporal lobes as well as the right frontopolar region occurred before the onset of asystole or bradycardia. The authors reviewed 81 cases of ictal bradyarrhythmia with simultaneous EEG scalp recording. In 70% of the cases, a temporal onset was identified, and in 30% a frontal onset was seen with an equal distribution between left and right hemisphere. In conclusion, seizure activity in the frontal, temporal and insular cortex was associated with ictal bradycardia and asystole. However, ictal bradycardia and asystole are of no lateralizing value and it remains unclear if seizure spread to any particular brain region is of relevance.
We report the case of a 41-year-old man who presented with refractory, right temporal seizures resulting in traumatic syncope as the sole ictal semiology.
Patients who fall as a result of a seizure or have generalized epilepsy are at particular risk of epilepsy-related injuries (Wirrell 2006). Additionally, falls cause increased utilization of health-care resources and negatively affect the quality of life (Hamer et al. 2006). The same applies to syncope-related traumatic injuries as they also occur due to the rapid loss of consciousness (Schuchert et al. 2005). Our case shows that insertion of a pacemaker may prevent future, traumatic falls.
Case report
A 41-year-old Caucasian male with a history of epilepsy since the age of 32, was referred for pre-surgical evaluation. The patient reported frequent falls with severe traumatic injuries, which had resulted in a basal skull fracture with a right temporal, subdural haematoma at the age of 39. Right fronto-temporal epileptiform discharges had been recorded in the past. The patient reported an initial feeling of weakness followed by syncope. Enuresis, encopresis, tongue biting, as well as prolonged postictal confusion were denied. The frequency varied between three times a week and one in 6 months, despite antiepileptic medication with carbamazepine (CBZ), gabapentin, topiramate and levetiracetam. At the onset of the epilepsy, two generalized tonic-clonic seizures had occurred. His prenatal history and developmental milestones were unremarkable. There was no history of febrile convulsions, meningitis or head trauma prior to the onset of epilepsy. There was no history of cardiac or thyroid dysfunction.At the physical examination no abnormalities were detected. The patient’s blood pressure was 130/75 mmHg, pulse was 76. The ECG showed a sinus rhythm of 85 bpm with no conduction or repolarisation abnormalities.
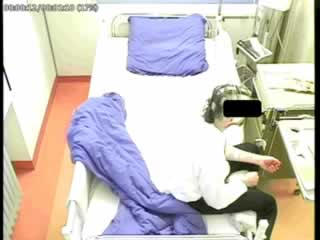
During a 96-hour, video-EEG monitoring, intermittent slowing and sharp waves were recorded over the right temporal lobe (maximum Sp2 and T8). Six, right temporal EEG seizures of 22-50 seconds duration were recorded, all of which were accompanied by an asymptomatic ictal bradycardia (figure 1). In two of these seizures, an ictal asystole of 10 seconds duration followed, which was accompanied by syncope during which the patient fell, hitting his head once (figure 2, see video sequence). No other ictal or postictal symptoms were observed.
High resolution magnetic resonance imaging (MRI) showed residues of the right temporal subdural haematoma and craniotomy, but did not reveal any further intracerebral lesions.
Ictal asystole was diagnosed and a cardiac pacemaker was implanted. Subsequently, the patient was directly readmitted to our video-EEG monitoring unit for a 24-hour recording. One, typical, right temporal EEG-seizure was recorded associated with ictal bradycardia inducing pacemaker activity. No syncope occurred and the patient remained unaware of the seizure (figure 3). Currently, at his nine-month follow-up, the patient reported no overt seizures, syncopes or traumatic falls. In view of the fact that patient seems unaware of his seizures and any pacemaker activity, we cannot exclude that the patient continued to have subclinical seizures.
Discussion
Ictal bradycardia and asystole may be of relevance in epilepsy patients presenting with syncope as well as traumatic falls, and are a potential contributor to SUDEP.The literature on ictal asystole is anecdotal and consists of case reports and small case series. Guidelines for the care of patients with ictal asystole are lacking. Insertion of cardiac pacemakers may prevent potentially traumatic syncopes and life-threatening cardiac arrests (Carvalho et al. 2004, Kowalik et al. 1998, Rugg-Gunn et al. 2004).
Our case uniquely demonstrates that implantation of a cardiac pacemaker, while continuing AEDs, may result in long-term freedom from syncope and traumatic falls. Over the follow-up time of nine months, the patient had not experienced any overt clinical seizures. Fortunately, the prevention of ictal asystole did not result in propagation of ictal activity and secondary generalized seizures as subclinical EEG seizures might persist unnoticed. Therefore, a case-by-case assessment should be performed. Informed consent on possible outcomes, including “no differences in seizure frequency” should be obtained (Mondon et al. 2002).
The case series reported by Ghearing et al. presents long-term results after pacemaker insertion. Only one out of seven implanted patients reported a seizure-related fall in a mean follow-up period of 27 months. Before implantation, all seven patients had experienced falls and unconsciousness during their focal seizures (Ghearing et al. 2007). As in our case, this series shows that pacemaker implantation may reduce morbidity in patients with symptomatic ictal asystole.
In future, severe trauma and possibly SUDEP may be prevented by early identification of high risk patients, resulting in early diagnosis of cardiac arrythmia. Our case conforms with current literature attributing ictal bradycardia to partial seizures and temporal lobe epilepsy (Britton et al. 2006, Leung et al. 2006).
Current hypotheses state that seizures may lead to stimulation of the insula, cingulated cortex, amygdala or hypothalamus, which regulate cardiac function through connections to brainstem and spinal cord nuclei (Britton and Benarroch 2006, Leung et al. 2007, Oppenheimer 2007). In patients at high risk of SUDEP, and a history of suspicious episodes, implantable loop recorders may identify potentially fatal, peri-ictal cardiac abnormalities (Rugg-Gunn et al. 2004).
Our patient received CBZ during the video-EEG monitoring, and CBZ has previously been reported to cause arrhythmia and to alter cardiac autonomic function (Timmings 1998). Therefore, a contribution of CBZ to the asystole cannot be ruled out. However, studies on CBZ in SUDEP and in ictal bradyarrhythmia are contradictory and have not found an association between the use of CBZ, or any other individual AED, and the aforementioned complications (Schmidt and Krämer 2006).
Our case demonstrates that ictal falls might be the only ictal semiology. Case series usually show auras or partial seizures occurring prior to ictal bradycardia and asystole (Schuele et al. 2007). Witnessed cases of SUDEP were principally accompanied by generalized seizures or partial seizures in only a few cases (Langan et al. 2000). Clinically, attacks of ictal asystole are usually associated with loss of muscle tone or brief, arrhythmic, bilateral upper extremity posturing and jerking that is distinct from the rhythmic and more sustained tonic-clonic activity or dystonic posturing typically seen in the context of seizures not complicated by cardiac arrhythmias (Ghearing et al. 2007).
A history of a sudden change in ictal semiology, including sudden falls in patients with focal epilepsy, should raise the clinician’s suspicion of ictal asytole.
Finally, the diagnosis of ictal asystole requires the recording of a representative clinical event during simultaneous video-EEG/ECG monitoring (Britton and Benarroch 2006). To identify patients at risk of ictal asystole, the ECG should be monitored for peri-ictal bradycardia within simultaneous EEG/ECG recordings of partial seizures.