Epileptic Disorders
MENUEpileptic negative myoclonus in herpes simplex virus encephalitis Volume 24, numéro 2, April 2022
Case study
A 74-year-old woman with no history of epilepsy presented to our satellite facility with acute mental status change and facial twitching, as a possible seizure. Brain MRI showed diffusion restriction in the left temporo-parieto-insular cortex with no subcortical involvement (figure 1A). Routine EEG showed a continuous focal seizure pattern in the left hemisphere with right facial clonic twitching. By hospital Day 2, she had also developed intermittent fevers. On examination, she was found to have a mixed aphasia with dysfluent speech and difficulty following complex commands. A repeat EEG performed on Day 3 showed left hemispheric lateralized periodic discharges (LPDs) and four focal seizures with right facial clonic twitching. She was treated with IV levetiracetam and lacosamide. The patient was then transferred to our hospital.
On arrival to the neurological ICU, she was emergently intubated for hypoxic respiratory failure. IV acyclovir was started along with broad-spectrum antibiotics. Continuous video-EEG monitoring showed left hemispheric LPDs and frequent focal electrographic seizures, consisting of paroxysmal theta and alpha rhythms. CSF showed 117 leukocytes/μL (57% lymphocytes), protein at 45 mg/dL and glucose at 85 mg/dL. CSF HSV1 polymerase chain reaction was positive, confirming the diagnosis of HSV encephalitis.
By Day 5, the bedside nursing staff reported seeing “twitching movements” of the right arm (video sequence). The EEG did not show electrographic seizures, but the movements appeared to be associated with the LPDs.
We placed surface EMG electrodes on bilateral biceps (BB1-BB2) and triceps (TR1-TR2) as well as on the right forearm flexor (FF1-FF2) and extensor (FE1-FE2) compartments (the analysis is discussed below).
The patient's EEG showed improvement over the next 3-4 days and the LPDs disappeared by Day 8, while sedation was weaned off. She remained on the same anti-seizure medications and acyclovir. A repeat MRI performed on Day 12 showed extensive FLAIR hyperintensity in the left temporal lobe, insula and posterior orbitofrontal region with very subtle change in the left thalamus (figure 1B). Her course was complicated by Takotsubo cardiomyopathy and hospital-acquired pneumonia. By the time of discharge, she had improved neurologically and was more awake and alert, and was able to follow simple commands with slight right motor neglect. She was discharged to a nursing home with a tracheostomy.
Results
The EEG was recorded using the 10-20 international electrode system with a Neurofax EEG-1200 (Nihon Kohden; Tokyo, Japan). The surface EMG electrodes were placed such that each pair covered agonists and antagonists. The electrodes on the same muscle belly were referenced to each other (e.g. BB1 to BB2).
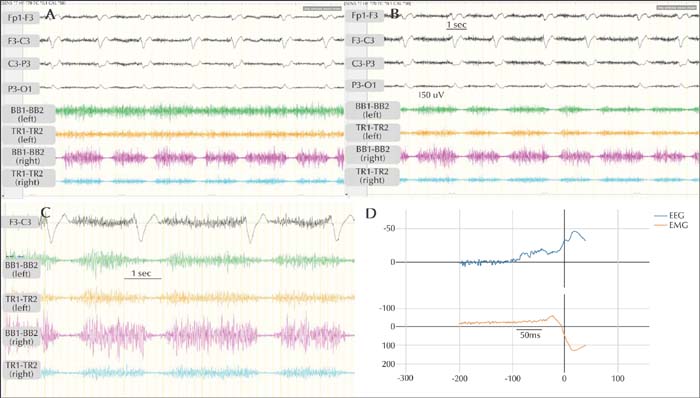
The duration of the LPDs varied from ∼550-650 ms (mean: 619.3±20.8 ms). The first negativity was consistently in the left parieto-central region. Clinically, the patient was sedated with propofol infusion. However, she had brief spontaneous and provoked arousals, especially when the sedation was withheld for the purposes of examination. In addition, levetiracetam and lacosamide were continued. During the quiet stages, there were no clinical manifestations associated with the LPDs. With arousals, the patient had diffusely increased muscle tone. The right arm EMG electrodes showed periodic interruption of the sustained tonic muscle activity (synchronized in agonists and antagonists), time-locked with the left hemispheric LPDs. These interruptions, defined as silent periods (SP), were occasionally synchronized in bilateral EMG electrodes, although they were more pronounced in the right arm (figure 2A, B).
Overall, the duration of the right arm SP was consistently longer than that of the left arm SP (figure 2C). Latencies between the EEG discharge and the EMG activity were calculated after back-averaging the polygraphic signal using BacAv, a freely available online platform (figure 2D). We used four EEG channels (including Fp1, F3, C3, P3, O1) and two EMG channels for back-averaging. To record latency of right temporalis/masseter SP, we used the right temporal channels as EMG channels (Fp2, F8, T8, P8). The results are summarized in table 1.
Overall, the latency from the onset of EEG activity to the SP was ∼55-60 ms for the right arm, ∼65-70 ms for the left arm, and ∼50-55 ms for the right temporalis.
Discussion
The phenomenon of ‘asterixis’ was first described by Adams and Foley in patients suffering from hepatic encephalopathy [1]. It consists of brief and involuntary jerks as the patient tries to maintain a steady posture. Polygraphic recording with EMG has shown that these jerky movements are produced by brief interruptions in the muscle tone [1, 2]. A similar phenomenon of brief muscular inhibition has also been reported in other disorders such as progressive myoclonic epilepsies and post-hypoxic action myoclonus. The term ‘negative myoclonus’ was first proposed by Shahani and Young to describe these periods of muscular inhibition [1].
Negative myoclonus can be subcortical or cortical [3]. Asterixis is a typical example of subcortical negative myoclonus. It is usually rhythmic with a frequency of 6-11 Hz and the SP duration is ∼50-200 ms [1, 3]. Cortical negative myoclonus is irregular and the SP duration is ∼100-400 ms [3]. An EEG transient preceding the cortical negative myoclonus may only be visible with an SP locked back-averaging technique [1, 3]. The SP in negative myoclonus may (type II) or may not (type I) be preceded by a positive myoclonic burst.
In 1968, Tassinari et al. described brief muscular inhibition in epilepsy patients which was related to a spike on EEG, referred to as “related epileptic silent period” (RESP) [4]. The duration of RESP varied widely from ∼40-120 ms to up to ∼200 ms [4], but the consistent feature was its temporal relationship to the spike rather than the slow wave [4].
The term RESP has been replaced by the phrase “epileptic negative myoclonus” (ENM) [1, 5]. ENM consists of brief interruptions in sustained muscle tone, time locked to an epileptiform discharge on the EEG in the contralateral sensorimotor cortex without any evidence of preceding positive myoclonus [1, 2, 5]. ENM is a non-specific motor phenomenon which has been described in a variety of chronic epilepsy syndromes [1, 5]. The typical duration of the SP in ENM is ∼100-400 ms and the typical latency from the beginning of the spike or an EEG transient is ∼20-40 ms [5].
As far as we are aware, this is the first case of ENM in a patient with HSV encephalitis. There have been anecdotal reports in the literature of patients with HSV encephalitis presenting with acute movement disorders such as chorea, ballism and myoclonus [6]. Negative myoclonus of subcortical origin has been reported in HSV encephalitis [6]. This patient's polygraphic recording showed brief SPs of ∼50-250 ms, present only in the left forearm extensor compartment without any preceding EEG discharge [6]. Our patient, on the other hand, had prolonged SPs, ∼400-600 ms, synchronized in right arm flexors and extensors and sometimes in bilateral arms, and all the SPs were time-locked with left hemispheric LPDs. Our patient did not have any MRI signal abnormality in subcortical structures on the initial MRI, whereas the patient reported in ref [6] had significant FLAIR hyperintensity early on in the right thalamus.
The SP duration and latency were longer in our patient compared to that reported in the literature.
Pathophysiology
The SP in ENM is thought to be due to an intrinsic cortical inhibitory process, centered in the contralateral sensorimotor cortex because of the location of the maximum activity of the spike [2, 3, 5, 7]. Transcranial magnetic stimulation fails to elicit a motor evoked potential (MEP) when applied during the ENM [1]. In patients with cortical reflex negative myoclonus, stimulation of the median nerve during sustained tonic contraction is associated with an enlarged N33 component of the somatosensory evoked potential, followed by SP lasting for ∼100-400 ms [3].
In healthy individuals, transcranial cortical electrical or magnetic stimulation over the sensorimotor cortex during sustained tonic contraction produces an SP following an MEP, lasting for ∼200-300 ms [8]. Studies have shown a positive linear correlation between the amplitude of the spike or the N33 component and the SP duration [3]. Similar correlation is seen between the intensity of the transcranial stimulation and the amplitude of the MEP and the following SP duration [8]. This is perhaps the reason why the duration of SP in our study is longer than that reported in literature, because of the very large amplitude of the LPDs.
The longer time latency between the LPDs and SP is likely related to the longer duration of the discharges themselves and the time it takes for temporal summation. The other possibility is that the inhibition is mediated by a subsequent subcortical mechanism. The intermittent bilateral nature of the negative myoclonus in our study could be attributed to the interhemispheric spread of the myoclonic discharge across the corpus callosum or a common subcortical mediator. We favor the former because the difference in latency between right and left arms was ∼10 ms, which is consistent with a transcallosal spread [9].
Multiple human studies in epileptic patients with intracranial subdural electrodes or stereotactic EEG have shown that the cortical negative myoclonus is likely generated in the premotor, primary motor, primary sensory and supplementary sensorimotor cortices [7, 10].
Supplementary material
Summary slides accompanying the manuscript are available at www.epilepticdisorders.com.
Disclosures
None of the authors have any relevant disclosures or conflicts of interest.