Epileptic Disorders
MENUEpilepsia partialis continua: aetiology, semiology and prognosis in a Spanish adult cohort Volume 18, numéro 4, December 2016
The definition and physiopathology of epilepsia partialis continua (EPC) has been broadly discussed and modified (Obeso et al., 1985; Cockerell et al., 1996), and this is presently still under debate. In a recent ILAE Task Force Report, EPC was considered a focal status epilepticus with motor symptoms as the most prominent (Trinka et al., 2015). However, some authors propose broader definitions that include sensory semiology (Mameniskiene et al., 2011).
In 1982, Bancaud et al. described two types of EPC. Type 1 (Kozhevnikov syndrome) implies direct damage to the rolandic cortex resulting from various different aetiologies, and type 2 (Rasmussen syndrome) corresponds to EPC caused by a chronic encephalitis of one hemisphere. Previous publications on EPC have included paediatric and adult populations, and hence both types are usually studied together. However, EPC type 1 is mostly seen in adults and studies focused on this type of EPC are uncommon.
The most prevalent aetiology in occidental adults is stroke (Thomas et al., 1977; Cockerell et al., 1996; Gurer et al., 2001; Pandian et al., 2002; Sinha and Satishchandra, 2007). In the oriental population, metabolic causes are more frequent, especially non-ketotic hyperglycaemia (Phabphal et al., 2012; Shrivastava et al., 2013), along with infectious diseases, which are also not uncommon in some geographical areas (Gurer et al., 2001; Pandian et al., 2002).
EPC is a pharmacoresistant entity (Thomas et al., 1977; Cockerell et al., 1996; Gurer et al., 2001; Pandian et al., 2002; Sinha and Satishchandra, 2007; Mameniskiene et al., 2011), and there is evidence pointing to a lack of response to benzodiazepines (BZDs) (Thomas et al., 1977; Gurer et al., 2001). No other antiepileptic drugs (AEDs) have been shown to be specifically effective as treatment for EPC.
Regarding prognosis, a shorter duration of episodes has been related to tumours or strokes (Thomas et al., 1977), and seizure recurrence was found to be lower in patients with metabolic causes (Phabphal et al., 2012). Nearly half of patients remain dependent in daily-life activities after an episode, and approximately one third are severely impaired (Pandian et al., 2002). Rasmussen's encephalitis has been identified with a worse prognosis with regards to seizure control and functional outcome (Sinha and Satishchandra, 2007). Finally, mortality that results directly from the disorder occurs in about 29% of patients (Gurer et al., 2001).
Given the few published series focused on EPC type 1, our study aims to examine the aetiology and long-term outcome of these patients.
Material and methods
A retrospective study was conducted including patients with adult-onset EPC (above 16 years old), diagnosed and followed in a tertiary centre between July 2006 and April 2013. These patients were selected from our epilepsy unit database, which includes all patients diagnosed with epilepsy during those years, either in the emergency room or in the hospital wards, and followed as outpatients. We also included, prospectively, patients diagnosed with EPC in the emergency room or in the hospital wards between April 2013 and February 2014. All patients were diagnosed by neurologists with expertise in epilepsy (MT, ES, and XS).
We collected demographic and clinical data, as well as information related to aetiology, neuroimaging, EEG recordings, and treatment. Except for cases with early death, all patients had a prospective follow-up period which was longer than six months.
We included patients who could be clinically defined according to Obeso et al. (1985): “spontaneous regular or irregular clonic twitching of cerebral origin sometimes aggravated by action or sensory stimuli confined to one part of the body and continuing for a period of hours, days or weeks”. We excluded patients with a follow-up period of less than six months and those with incomplete information for analysis.
An expert neuroradiologist (SS) performed a second review of structural neuroimaging (CT or MRI), with special emphasis on motor cortex involvement. EEGs were recorded with surface electrodes using the standard protocol, according to the 10-20 international system; we classified EEGs as ictal or interictal, and recorded the presence of epileptiform discharges (EDs).
EPC was classified into two groups, according to seizure course during an acute episode:
- –Continuous: continuous clonic muscular twitches during an EPC episode, which could also include rhythm variation.
- –Intermittent: clonic muscular twitches that disappeared for a minimum of one hour, but relapsed within less than 24 hours from the seizure onset.
We defined recurrence as the reappearance of EPC after a minimum of one week with no seizures.
We categorised patients with EPC depending on the number of body areas involved: with either a single body area (one limb, face or trunk) or multiple body areas (more than one area). Aetiology was classified according to the ILAE New Status Epilepticus Classification on acute, chronic or progressive status (Trinka et al., 2015).
Prognostic groupswere considered as follows:
- –Favourable prognosis: patients who did not have a relapse of EPC or who remained non-epileptic after EPC.
- –Unfavourable prognosis: patients who developed epilepsy after EPC or those who had a relapse of EPC.
- –Death: occurring early during EPC, or late after EPC resolution.
Statistical analysis was performed using SPSS Statistics 17.0 version for Windows. For the comparison of categorical variables, we used the Pearson's Chi-square test or Fisher's exact test. Comparisons of the continuous variables between two groups of subjects were performed using the Student's t-test and Mann-Whitney U test; the latter was used when the values showed a non-normal distribution (number of AEDs and duration of EPC); comparisons of variables between more than two groups and continuous variables were analysed using ANOVA or the Kruskal-Wallis test. We performed ROC curves in order to establish optimal cut-off points during the duration of EPC, in order to predict the best prognosis of patients. A Cox regression model, adjusted by aetiology, was performed to assess the relationship between status duration and mortality. During follow-up, the Kaplan-Meier test was used in order to analyse EPC recurrence. We considered statistical significance at p<0.05.
Results
We investigated 27 patients; 20 retrospectively and seven prospectively. During the retrospective recruitment, five patients were excluded due to insufficient available data, and one had less than six months of follow-up. Demographic characteristics are shown in table 1. Median follow-up was 17 months (range: 6-69 months) and median age at EPC presentation was 63 years old (interquartile range: 56-79 years old).
Patients with previous epilepsy (n=11) included structural epilepsies in eight cases (two ischaemic strokes, two haemorrhagic strokes, three CNS tumours, and one progressive multifocal leukoencephalopathy).
Clonic jerking affected multiple body areas in 55.6% (n=15). For EPC affecting a single body area, the arm was the area most likely affected (22.2%; n=6), with a predilection for distal muscles. The most common course of EPC was intermittent (63%; n=17) and duration was longer than 48 hours in 71.3% (n=19) of patients. Fifty-one percent of patients (n=14) had associated neurological deficits; motor weakness (29.6%; n=9) was the most frequent; other symptoms were somatosensory, aphasia and oculocephalic deviation. Other seizures coexisted with EPC in 22.2% of patients; motor symptoms were always the most prominent. The clinical characteristics are detailed in table 2.
An EEG was performed within the first 72 hours in 21 patients. We obtained 13 ictal EEGs (table 3). EDs were seen in 47.8% of EEGs, most frequently at the central contralateral electrodes (72.7%). EDs were detected ictally in 76.9% EEGs. All patients with EPC affecting multiple body areas showed EDs on EEG, compared to 30.8% of patients with single body area involvement (p=0.001).
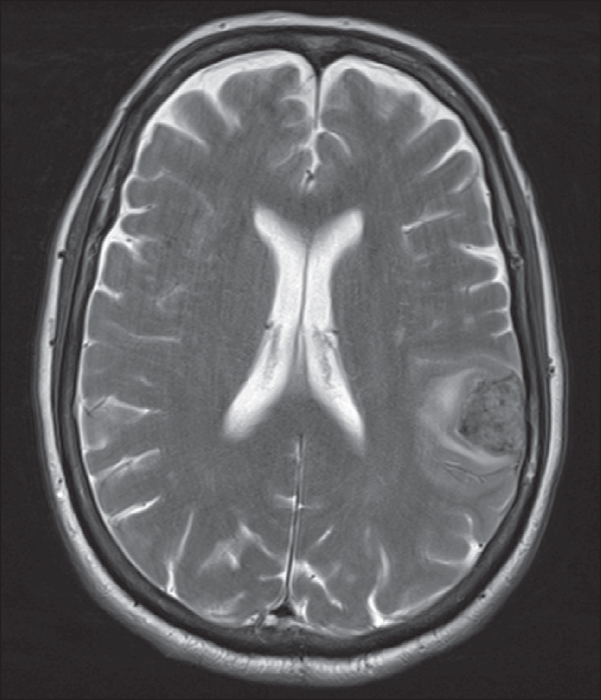
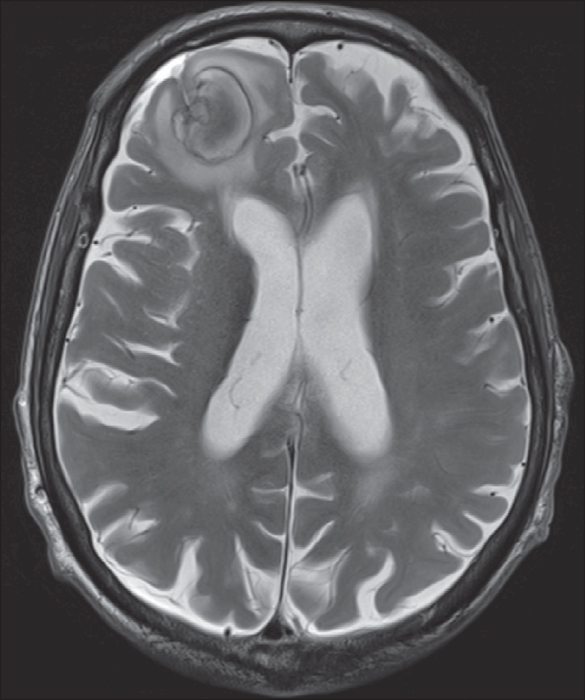
Neuroimaging was obtained in all patients (table 4); 77.8% showed a lesion, which was cortical in 70.4%. These cortical lesions involved the primary motor cortex in 73.68%, i.e. 51.8% of the total sample.
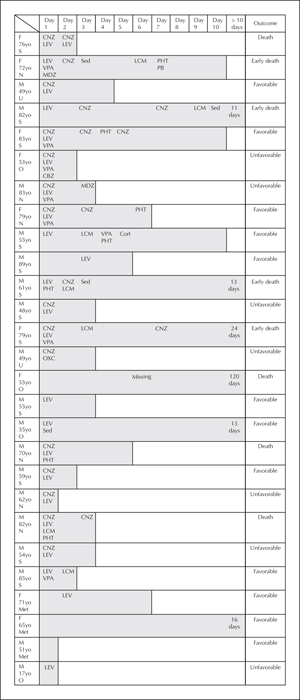
Aetiologies are detailed in table 1. EPC was acute or progressive symptomatic in 81.4% and only three patients had a remote cause. The most frequent cause of EPC was stroke (44.4%), followed by CNS tumours (21.4%). Non-ketotic hyperglycaemia was the cause for the three EPC cases with metabolic disorders, which were resolved after correction of the metabolic disturbance. In patients with established epilepsies (40.7%), an acute cause of EPC, independent of the underlying disease, was found in 54.5% (n=6); these acute causes were stroke (n=3), CNS tumour (n=1), and post-radiation syndrome (n=2). A second-line therapy was required in 89.9% of patients. BZDs were the first-line therapy in most cases, followed by conventional AEDs. In terms of therapeutic lines, 81.4% had refractory status epilepticus, as a second-line therapy failed to control seizures. Half of patients received more than two AEDs, in addition to BZD (average of 2.6), and 18.5% required sedation and admission to an intensive care unit (figure 1).
Considering the overall duration of EPC, the optimal cut-off period to predict risk of death was nine days (with a sensitivity of 62.5% and specificity of 75%; positive predictive value of 50% and negative predictive value of 83.3%). After adjustment by multivariate analysis with aetiology, EPC duration was an independent predictor of mortality (p=0.034). In this group of patients, we observed a higher proportion of multiple body area involvement (88.9% vs 38.9%; p=0.04) (figure 2), as well as more AEDs used (median: 3; [IQR 2.5-5.5] vs 2 [IQR 1-3]; p=0.006). We observed 13 patients who responded to treatment within 72 hours, but there were no clinical predictors of early treatment response.
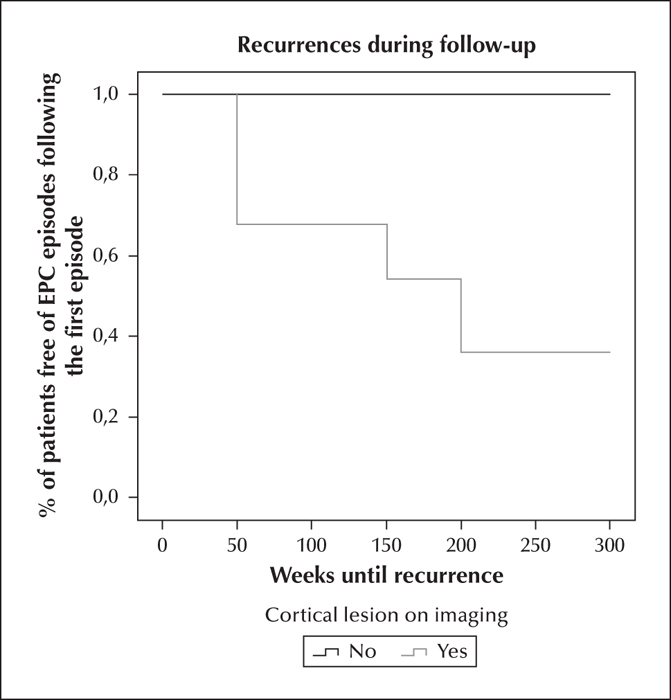
Favourable prognosis was seen in 48.1% patients, unfavourable prognosis in 25.9%, and 25.9% of patients died, half of them during the EPC episode (figure 2). Recurrence occurred in 25.9% of patients, and all of the recurrent events had similar clinical characteristics to the first episode, regarding semiology and duration. The median time to recurrence was 6.25 months (range: 1 week to 38.7 months); 63.6% of the recurrent events occurred during the first year of follow-up. Patients with cortical lesions on MRI showed a higher relapse rate (p=0.037); a cumulative recurrence risk of 60.2% was identified in lesional cases, compared to zero recurrences in non-lesional patients over a three-and-a-half-year period (figure 3).
Discussion
There is presently no agreement regarding EPC definition, classification, or semiology. Its relatively low prevalence has led to retrospective studies, generally with small sample sizes, which have contributed to a lack of consensus on EPC characterisation. Our study showed that most episodes of EPC in adults are acute symptomatic, usually secondary to stroke, with mortality affecting close to one third of patients, which is similar to previous publications on EPC in adults (Thomas et al., 1977; Cockerell et al., 1996; Gurer et al., 2001).
Regarding EPC duration, there are wide differences among studies, with averages ranging from three days to 6.5 years (Mameniskiene et al., 2011; Shrivastava et al., 2013), probably related to the inclusion of different paediatric and adult populations. The average duration in our sample was 4.5 days, which is in line with studies in which only adults were included (Phabphal et al., 2012; Shrivastava et al., 2013). Mameniskiene et al. described different EPC subtypes based on the time of evolution and seizure pattern. Their series included paediatric population with sensory seizures, but excluded post-stroke patients, which corresponds to a fairly different population profile compared to the patients in our study (Mameniskiene et al., 2011). The distinct courses and duration in our study are probably related to these differences.
Unlike the study of Thomas et al. (1977), which also focused on EPC semiology and duration to identify predictor factors, we found a higher risk of mortality and a trend towards a poorer pharmacological response in patients with multiple body area involvement. Population differences and treatment availability may explain these discrepancies.
As seen in our patients, stroke and tumours are reported to be the most common causes of EPC described in adults (Thomas et al., 1977; Cockerell et al., 1996). Although few studies have analysed EPC prognosis in relation to underlying pathology, aetiology is accepted as the principal prognostic factor for EPC, e.g. metabolic causes are associated with better seizure prognosis and survival (Phabphal et al., 2012). In our study, we integrated the new ILAE aetiological classification for status epilepticus, however, we found no differences among groups (Trinka et al., 2015). On the other hand, patients with cortical lesions on neuroimaging showed more relapses compared to non-lesional patients.Notwithstanding, we must take into consideration that the minimum follow-up period was six months, and that recurrent events could appear later.
Based on the literature, long duration of status epilepticus is known to be a risk factor for mortality (Neligan and Shorvon, 2011), and a relation between EPC duration and prognosis has been described (Pandian et al., 2002; Phabphal et al., 2012). In accordance, we observed that adult patients with EPC lasting longer than nine days were more likely to die. Although our study was based on a small sample, we performed a multivariate analysis which showed that EPC duration was an independent predictor of mortality. This finding should be further evaluated using a larger and more heterogeneous cohort, with a lower percentage of aetiologies with high short-term mortality.
Based on a single study including eight necropsies, EPC was related to lesions in the primary motor cortex or immediately adjacent areas (Thomas et al., 1977). In contrast, only nearly half of our patients, with different types of lesions, showed direct involvement of the primary motor cortex, as analysed by MRI. This finding suggests that the propagation of epileptic activity from distant areas to a non-impaired primary motor cortex can occur during some episodes of EPC.
EPC manifested mostly as an acute symptomatic status, and appeared both within the context of previous epilepsy or previous injury of the CNS. Thus, the presence of both conditions does not rule out the possibility of a new onset aetiology being the cause of EPC, despite the underlying previous epilepsy or brain injury. Therefore, we encourage a full aetiological study in all cases of EPC.
Consistent with previous series, a high rate (85%) of drug refractoriness was seen in most patients. The response to BZD was absent or only transitory. Among the different AEDs, none of them showed a superior effect (Thomas et al., 1977; Cockerell et al., 1996; Gurer et al., 2001; Pandian et al., 2002; Sinha and Satishchandra, 2007; Mameniskiene et al., 2011).
The main limitation of our study was the retrospective design, which may have inferred a selection bias and probably accounts for the differences in recruitment between the first years and the last year of the study. Although our population size was considerable for a condition with low prevalence, along with EEG and neuroimaging assessment, compared to previous reports, it may not have been possible to obtain clinical significance for some variables due to the relatively small sample size.
Conclusion
The most frequent aetiology of EPC in our patients was stroke. Multiple body area involvement and duration were associated with mortality. Patients with cortical lesions had more EPC relapses during follow-up.
Supplementary data
Summary didactic slides are available on the www.epilepticdisorders.com website.
Disclosures
The authors have no conflict of interest to declare.