European Journal of Dermatology
MENUExpert opinion: defining response to omalizumab in patients with chronic spontaneous urticaria Volume 27, numéro 5, September-October 2017
Chronic spontaneous urticaria (CSU) is defined as the spontaneous occurrence of wheals (hives), angioedema, or both, lasting for six weeks or more with no known specific trigger [1]. Hives are characterized by a central swelling of variable size, usually surrounded by a reflex erythema, and are typically associated with intense itching. Angioedema is identified as a sudden, pronounced swelling of the lower dermis and subcutaneous tissue, commonly with mucous membrane involvement. It is often accompanied by pain or burning rather than itching [1]. As well as the clinical burden associated with the signs and symptoms of hives and angioedema, CSU impacts many other facets of patients’ daily lives. Indeed, factors such as the unpredictability of attacks, social isolation, and reduced sleep quality due to itching and associated fatigue all contribute to a diminished quality of life (QoL) [2-4]. Moreover, CSU bears a socioeconomic burden due to direct (medication, healthcare visits) and indirect (reduced work productivity, increased work absenteeism) healthcare costs [2, 5].
The EAACI/GA2LEN/EDF/WAO international urticaria guidelines recommend second-generation H1-antihistamines, at licensed doses, as first-line treatment for CSU, and in those patients not responding to therapy, at up to four times the licensed dose as second-line treatment [1]. However, licensed doses of H1-antihistamines lead to absence of symptoms in fewer than 50% of patients with CSU [1, 2, 6-8], and up-dosing can increase the efficacy of H1-antihistamines in some, but not all patients [6, 7, 9-12]. A recent systematic review and meta-analysis regarding up-dosing of non-sedating antihistamines in patients with CSU found the proportion who responded to antihistamine up-dosing was 63.2%, with 38% of patients responding to licensed doses. Moreover, the authors found that up-dosing appears to lead to a significant improvement in the itch control component of CSU, but not the number of hives [12]. In cases where patients do not respond adequately to increased doses of H1-antihistamines, the international urticaria guidelines recommend either omalizumab, ciclosporin, or montelukast as third-line add-on treatment [1].
Omalizumab is a recombinant, humanized anti-immunoglobulin (Ig)-E antibody, approved as add-on therapy for the treatment of CSU in adults and adolescent (12 years and above) patients with inadequate response to H1-antihistamine treatment [13, 14]. Omalizumab is the only licensed treatment for use as third-line therapy for CSU. By binding to free IgE, omalizumab prevents it from binding to high-affinity receptors (FcεRI). This ultimately reduces the signs and symptoms of urticaria [15]. Notably, it is likely that omalizumab achieves its therapeutic effects through various different pathways [16, 17], and as such its mechanism of action in CSU has yet to be fully elucidated. Approval of omalizumab as third-line treatment for CSU was based on the results of three Phase III, multicentre, randomized, double-blind, placebo-controlled studies, namely ASTERIA I, ASTERIA II, and GLACIAL [18-20]. Together, these pivotal trials demonstrated that omalizumab, at 300 mg, provided significant and sustained improvement versus placebo for the symptoms of CSU with an inadequate response to H1-antihistamine treatment. Omalizumab treatment resulted in a significantly reduced weekly Itch Severity Score (ISS) between baseline and Week 12 (ASTERIA I, ASTERIA II and GLACIAL) and baseline and Week 24 (ASTERIA I and GLACIAL). Moreover, no new safety concerns were raised with omalizumab in this patient population relative to its known safety profile for allergic asthma [15, 18-20].
The licensed dose of omalizumab is 300 mg in the EU and either 150 or 300 mg in the US [13], by subcutaneous injection every four weeks. Some reports have shown examples of the optimization of omalizumab treatment in patients who show inadequate response by increasing the dose or decreasing the dosing intervals [21, 22]. There is currently no internationally agreed algorithm for the individualized management of omalizumab treatment, although a Danish algorithm was published in 2014, which was dependent on the 7-Day Urticaria Activity Score (UAS7) [23]. In addition, in patients who respond to omalizumab treatment, a consensus is needed on when and how to stop omalizumab. Moreover, there is no agreement on the definition of a non-responder and when to admit this status and consider other treatment options.
The Urticaria Activity Score (UAS) is a validated measure for assessing urticaria activity in patients with CSU [24]. EAACI/GA2LEN/EDF/WAO international urticaria guidelines recommend evaluation of disease activity and response to treatment in routine clinical practice using the UAS7 (the sum of the UAS over seven consecutive days) [1]. However, differences exist with regard to how CSU treatment response is measured and defined in both clinical trials and real-world clinical practice [18, 20, 25-32]. This lack of consensus on the definition of response to treatment potentially impacts perceived treatment efficacy, as well as patient satisfaction and subsequent compliance with therapy. Improved characterization of response to treatments such as omalizumab may help healthcare providers to better convey realistic expectations of the likelihood of a response to treatment at different time points [33].
A meeting of 12 chronic urticaria expert physicians was convened on 8th October 2015 in Copenhagen, Denmark, with the following objectives: (1) to reach a consensus on how response to treatment should be measured in clinical trials and real-world clinical practice; (2) to develop definitions of response and non-response to treatment in patients with CSU; and (3) to gain insight into the characteristics of responders and non-responders (or response patterns) to omalizumab and the practical implications of response and non-response, as well as different response patterns. The aim of this article is to provide a summary of key discussion points from the meeting regarding definitions of treatment response, modes of response/response patterns, factors affecting response, and a potential omalizumab treatment approach based on response patterns.
Definitions of response
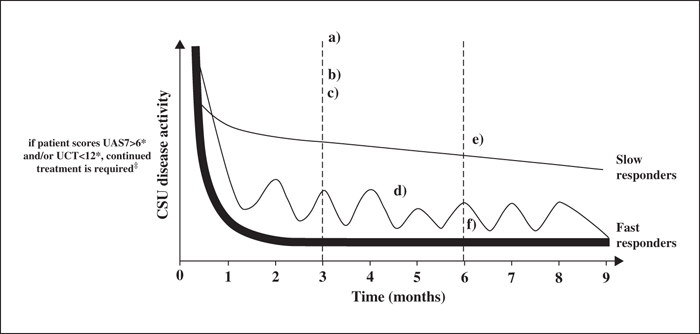
Given variations in the definition of response to treatment in CSU among physicians globally, it was agreed that there is a need for clarity regarding how “complete”, “partial” and “non-response” are defined. It should be noted, however, that the differing expert definitions of response broadly align on the same ultimate goal of complete control of urticaria signs and symptoms as rapidly and as safely as possible. A disease measurement tool is required when defining treatment response, supplemented by a patient-orientated measure (including patient expectations and QoL, such as the Chronic Urticaria Quality of Life questionnaire [CU-Q2oL] or Dermatology Life Quality Index [DLQI]). Further, UAS7 should be assessed every week during omalizumab treatment. This is particularly important during the first 12 weeks, when it would be possible to define a slow, fast, or partial response pattern (figure 1). Once the responder profile is defined, we believe that the UAS7 should be measured again the week before receiving omalizumab. Likewise, the Angioedema Activity Score (AAS) should be recorded for four consecutive weeks. This is important since patients suffering from CSU could have days with severe urticarial lesions, without swelling, and vice versa.
The UAS7 provides a validated composite of the ISS and number of hives score over a period of seven days (score ranges from 0 to 42), providing semi-quantitative information on disease activity between clinic visits [34]. A UAS7 of 0 indicates that the patient is itch- and hive-free, i.e. a complete response. A UAS7≤6 has been proposed to reflect well-controlled disease. UAS7 ranging from 7-15, 16-27, and 28-42 are proposed to indicate mild, moderate, and severe disease activity, respectively. These cut-off values appear to efficiently describe CSU health states with regards to signs and symptoms of the disease [35]. However, across studies employing the UAS7 tool, response to treatment has been defined differently (table 1) [18, 20, 25-32]. Some studies have defined treatment response as a UAS7 of 0, to indicate absence of itch and hives [30], although this may be difficult to achieve in real-life clinical practice and may not make a clinical difference in terms of QoL compared to a UAS7 of 1-6. Furthermore, individuals without CU are likely to experience itch occasionally, therefore having UAS7>0, but no urticaria. As such, a UAS7≤6 is proposed as a definition of CU treatment response.
The UAS7 does not include a component relating to angioedema and it is thought that the rate of angioedema may be under-reported in clinical trials. More than 50% of patients with CSU have associated angioedema at baseline and the presence of angioedema has been shown to impair QoL more than hives alone [36-39]. Omalizumab has been shown to be effective in treating CSU with associated angioedema. For example, a subgroup analysis of the Phase III GLACIAL trial demonstrated that omalizumab treatment was associated with a reduction in angioedema compared with placebo [37]. Similarly, the X-ACT study in CSU patients with frequent angioedema, who had an inadequate response to H1-antihistamines at increased doses, found that following omalizumab treatment, the number of angioedema burdened days was 14.6 days compared with 49.5 days in the placebo group [40]. Overall, therefore, a definition of treatment response that incorporates angioedema would be useful.
The Urticaria Control Test (UCT) is a validated instrument designed to assess the level of disease control in patients with CU and thus aid treatment decisions [41, 42]. The test comprises four questions around how much patients have suffered from urticaria symptoms, how much symptoms have affected quality of life, how often treatment was not enough to control symptoms, and how well patients felt their urticaria was under control, over the past four weeks. The answer to each question is rated from 0 to 4, resulting in a total score ranging from 0 to 16. A UCT score of 0 indicates “no disease control”, a score of ≤11 indicates poor disease control, and a score of ≥12 points to well-controlled CU. The highest possible score of 16 represents complete disease control [41, 42]. It should also be noted that the UCT score comprises pruritus, wheals, and/or angioedema.
The DLQI is a validated patient questionnaire for evaluating health-related QoL (HR-QoL) in patients with a variety of skin conditions, including CSU. The DLQI consists of 10 questions across six domains: symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment. Impact of CSU on each factor is scored from 0 (not at all) to 3 (very much) and scores are totalled to give an overall DLQI score from 0-30. The minimum index scoring, from 0 to 1, corresponds to no effect at all on the patient's life and the maximum, from 21 to 30, to an extremely large effect on the patient's life [43-45].
The validated CU-Q2oL was specifically designed for the assessment of HR-QoL in CU, including the physical, psychosocial, and practical aspects of this condition. Twenty-three questions cover six key CSU-specific domains: itch, swelling, impact on life activities, sleep problems, “looks”, and limits. The overall score ranges from 0-100 with a higher score indicating greater impairment in HR-QoL [46].
In addition to using validated instruments, many physicians will base their definitions of treatment response on prior experience and clinical judgement. Some physicians may augment this by using real-life-orientated measures, such as the Physician's Global Assessment (PGA). In a recent retrospective analysis that evaluated the outcome of omalizumab treatment in 154 patients with CU, treatment response was graded according to a modified PGA, whereby complete or almost complete response corresponded to ≥90% reduction in symptoms, partial response to a reduction of between 30% and 89%, and no or limited response to a reduction of <30% [47].
We believe it would be valuable to conduct a responder analysis that separates response defined by ISS and number of hives (the individual components of UAS7), UAS7, and a QoL index, such as CU-Q2oL or DLQI, in order to better define treatment response in patients with CSU. A similar analysis for the four items of the UCT would also be helpful in this regard. Furthermore, since the presence of angioedema is often under-reported in clinical trials, it would be worth considering the addition of one line to the UAS7 to report the occurrence of angioedema. This could be a score of 0 (no angioedema in the previous 24 hours) or 1 (patient experienced angioedema in the previous 24 hours) that would be considered in addition to the patient's total UAS7. Such an approach would of course require further study for the purposes of validation. Alternatively, AAS should be used as a validated measure of angioedema activity [48].
Modes of response/response patterns
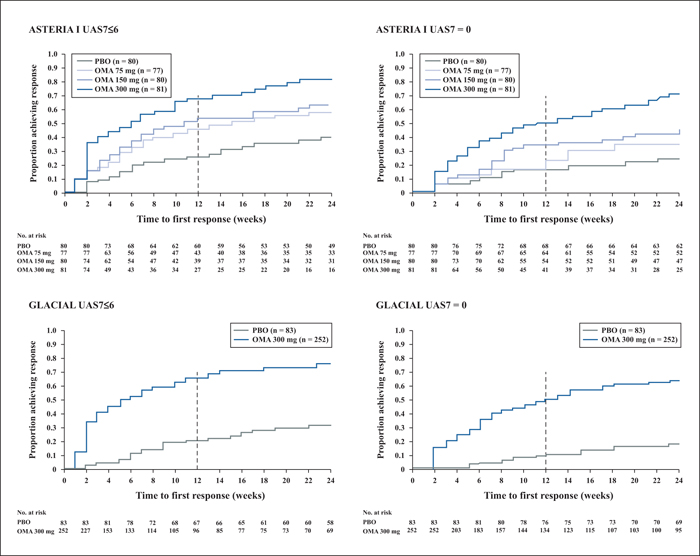
A recent publication provided more insights into data from the three pivotal clinical trials of omalizumab in 975 patients with CSU [33]. Patients were randomized to either placebo or omalizumab (75, 150 or 300 mg in ASTERIA I [n = 318; 24 weeks] and ASTERIA II [n = 322; 12 weeks]), or 300 mg in GLACIAL [n = 335; 24 weeks). Response was defined as well-controlled urticaria (UAS7≤6) or complete response (UAS7 = 0). Results of the analysis showed that omalizumab at 300 mg over six months was associated with the highest response rates (both well-controlled and complete) (figure 2). Additionally, median times to achieve well-controlled urticaria with omalizumab at 300 mg were three weeks in ASTERIA II and six weeks in ASTERIA I and GLACIAL, compared with seven weeks in ASTERIA II and 11 weeks in ASTERIA I with omalizumab at 150 mg [33].
The authors of the analysis suggested that the timing of attaining well-controlled urticaria or a complete response indicated there were potentially two categories of responder to omalizumab, i.e. “fast responders” (within Weeks 4-6) and those who required more than three doses to respond (“slow responders”). The finding that some patients with CSU responded to omalizumab treatment early while others took longer to achieve a response (Weeks 12-16) suggests that stopping treatment before three months may be too early and potentially misses an opportunity to control CSU symptoms in those patients who take longer to respond [33].
In the real-world setting, similar response rates with omalizumab treatment are observed across studies despite differences in definitions of response (table 1). Furthermore, the response rates achieved in these real-world studies are generally better than those seen in clinical trials.
Factors affecting response
The ability to predict response to omalizumab treatment for CSU would be highly beneficial since this would aid treatment decisions in clinical practice. In a recent retrospective study, a number of factors were found to be associated with an improved response to omalizumab [47]. Notably, CSU was associated with a higher proportion of complete or almost complete responders compared with chronic inducible urticaria, as assessed by PGA grading (67.4% vs 52.9%, respectively). Furthermore, among the patients with CSU, a greater proportion of complete/almost complete responders: had a negative histamine release test (77.3% vs 27.3%; odds ratio [OR]: 9.07; p<0.001); did not have angioedema (72.3% vs 56.1%; OR: 2.05; p = 0.066); and had no prior history of treatment with systemic immunosuppressants (71.4% vs 56.8%; OR: 1.91; p = 0.105). Moreover, there was a tendency for improved treatment response with older age at onset and a shorter disease duration [47].
With regards to a potential biomarker for predicting patient response to omalizumab treatment, a lack of basophil CD203c-upregulating activity in the serum of patients with CU has been found to correlate with clinical response to omalizumab [49]. In a retrospective study of 41 patients with CU with inadequate response to H1-antihistamines, CD203c-upregulating activity was present in 18 patients. Of these, nine patients (50%) demonstrated improvement with omalizumab treatment. In the 23 patients with no CD203c-upregulating activity, 20 (87%) had a response to omalizumab treatment [49]. Recently, Gericke et al. [50] found that basophil histamine release induced by CSU sera seems to correlate with a slow response to omalizumab, and may represent a future biomarker. Although this remains to be confirmed in larger, prospective studies, a lack of basophil CD203c-upregulating activity may be a clinically useful biomarker of response to omalizumab treatment in patients with CU. At present, there is insufficient evidence regarding which biomarkers may be predictive of response to omalizumab treatment.
According to Metz et al., the majority of patients with CSU showing a complete response to omalizumab treatment suffer a relapse (reoccurrence of symptoms) within 2-8 weeks of receiving their last omalizumab injection [51]. In this retrospective analysis, there was evidence that re-treatment with omalizumab is effective in this patient population [51]. Overall, 100% (n = 25) of patients achieved a complete response, defined as ≥90% improvement in UAS7, to omalizumab retreatment (doses ranged from 150 to 600 mg/month, in 2- to 4-week intervals). This finding is important because relapse following treatment discontinuation was seen in the Phase III clinical trials and commonly occurs in real-world clinical practice. Similar results regarding response to omalizumab re-treatment have been observed by Labrador-Horrillo and colleagues [31] and by Ghazanfar and colleagues [47]. Any algorithm for the management of omalizumab treatment would have to take relapse and re-treatment into account.
Assessing non-response/recommended treatment algorithm
In our opinion, patients should be treated for at least six months, before concluding that they are a non-responder to omalizumab treatment. As noted in the analysis by Kaplan et al., timing of response to omalizumab may vary, with some patients being fast responders (within 4-6 weeks) and others taking longer to start to respond (Weeks 12-16) [33]. Based on these observations, it would seem reasonable to suggest that treatment with omalizumab at 300 mg should be continued in patients showing limited response at 12 weeks in order to maximize the possibility of achieving symptom control.
A complete response could be achieved in patients with CSU who are partial responders to third-line omalizumab by individualizing the treatment regimen, e.g. increasing the dose of omalizumab or reducing the dosing interval. In this regard, different treatment regimens may be required for the different types of omalizumab responder, i.e. fast, slow, or any other responder subgroups identified. Our proposed potential approach for omalizumab management for complete, partial and non-responders to treatment is shown in figure 2.
Prior to starting omalizumab treatment, it is important to exclude certain differential diagnoses of CSU, for example urticarial vasculitis and urticarial eruptions in auto-inflammatory syndromes. This should be completed as part of the guideline-recommended diagnostic algorithm [1].
During the first 1-3 months of treatment with omalizumab, we believe that it is important to define the response profile of the patient. In cases where there is no response during the first 1-3 months, physicians should consider reassessing the original diagnosis before attempting up-dosing or continuing treatment with omalizumab. If a primary diagnosis of CSU is confirmed, omalizumab treatment should be continued at the same or higher dose as judged by the individual clinician, in line with patient expectations, and according to local guidelines. Where a partial response to treatment is seen, physicians should first ensure that the patient has not stopped taking his/her H1-antihistamine treatment (occasionally, in our experience following initiation of omalizumab, when patients start to see a response they may decide that they no longer need to take antihistamines in addition to omalizumab). Following this, physicians should consider optimizing the omalizumab dose or dosing intervals, or both. Patients’ variation in response between doses should also be taken into account. For example, some patients may experience a relapse of CSU symptoms towards the end of the four weeks between omalizumab doses. In this patient population, it may be useful to consider shortening omalizumab dosing intervals.
After 3-6 months of treatment, in cases where a partial response is seen, physicians should consider reassessing the diagnosis (if the diagnosis has not previously been reassessed). As before, if a primary diagnosis of CSU is confirmed, omalizumab treatment should be continued and the individual clinician should judge whether to increase the dose. If a complete response has been present for 3-6 months, we advise physicians to consider reducing the dose of omalizumab and/or increasing the dosing intervals, or discontinuing omalizumab to assess for spontaneous remission. Results from the ongoing Phase III OPTIMA (Efficacy of Optimized Re-treatment and Step-up Therapy With Omalizumab in CSU Patients) study are expected to clarify optimal omalizumab dose [52].
If there is no response to omalizumab after six months of treatment, we would advise considering the patient to be a non-responder, discontinuing omalizumab and considering an alternative treatment option.
In treatment responders, omalizumab treatment can be resumed at a later stage after discontinuation with the same degree of symptom control [51]. A clear algorithm for when to restart treatment is needed. If patients have a UAS7>6 and/or UCT<12 (indicating active disease and/or poor disease control) during follow-up, then continued treatment is advised. This is also dependent on physician judgement and the patient's own expectations. For example, depending on their previous disease activity, some patients may be content to live with moderate CSU and their physician may propose to restart treatment when their UAS7 is >16. The presence or absence of angioedema should also be considered, as described previously when evaluating response and managing treatment.
Key areas for future research
Clinical trials and real-world studies that support the efficacy and safety of omalizumab treatment in patients with CSU with an inadequate response to H1-antihistamines continue to accumulate. Nevertheless, many important questions regarding the use of omalizumab remain to be answered in order to optimize treatment management and patient outcomes.
In particular, further investigations into predictors of good outcome, optimal dose, and dosing intervals based on treatment response to omalizumab in CSU are needed. We define outcome as an objective treatment effectiveness measurement. There is also an urgent need to clarify the options on how to treat non-responders. In addition, further studies to determine the optimal duration of therapy and long-term treatment effects, in terms of efficacy and remission rates and the safety/tolerability profile of omalizumab, are required, as well as studies to determine the best strategy for discontinuing omalizumab. Finally, it is important to harmonize patient/physician evaluation and definition of treatment response globally, across countries, for improved comparison of real-world data.
Acknowledgements
This review summarizes discussions from a meeting that took place in Copenhagen, Denmark in October 2015. The publication presents the views of the authors and not Novartis. Editorial assistance was provided by Sharon Smalley, David Steele, and Jane Blackburn, professional medical writers contracted to CircleScience, an Ashfield company, part of UDG Healthcare plc. Writing support was provided by Novartis Pharma AG.
Disclosure
Financial support: This review is supported by Novartis Pharma AG, Basel, Switzerland. Conflict of interest: Marta Ferrer has received research support from Novartis; consultancy fees from FAES, Genentech, Inc, and Novartis; and lecture fees from MSD and Menarini. Isabelle Boccon-Gibod has received consultancy fees and honoraria from Novartis for participation in advisory boards and presentations, and financial contribution to research projects. Margarida Gonçalo has received consultancy fees from Novartis and lecture fees from Janssen Pharmaceutica and Novartis. Hüseyin Serhat İnalöz has received honoraria from Novartis, MSD, AbbVie, Pfizer, Eli Lilly, Roche, the Menarini Group, Deva, and Erkim. André Knulst has received payment from Novartis for participation in advisory boards, giving presentations, and financial contribution to research projects. Hilde Lapeere has received payment from Novartis for participation in advisory boards. Anna Tagka has received lecture fees from Novartis. Fernando Valenzuela has received payment from AbbVie, Eli Lilly, Janssen, Novartis, and Pfizer for participation in advisory boards and research projects. Jensen Yeung has been a speaker, consultant, and investigator for AbbVie, Allergan, Amgen, Astellas, Baxalta, Boehringer Ingelheim, Celgene, Centocor, Coherus, Dermira, Eli Lilly, Forward, Galderma, Janssen, Leo, Medimmune, Novartis, Pfizer, Regeneron, Roche, Takeda, Valeant, and Xenon. Simon Francis Thomsen has received research support, consultancy fees, and lecture fees from Novartis.