Epileptic Disorders
MENUThe localizing value of epileptic auras: pitfalls in semiology and involved networks Volume 21, numéro 6, December 2019
An aura is usually considered to be the onset of a seizure occurring before the alteration or loss of awareness, but should be distinguished from a premonitory or prodromal sensation because the auras occur at seizure onset, while prodromal experiences occur significantly before the seizure, usually at least 30 minutes. Moreover, excluding motor symptoms, auras are often restricted to subjective experiences (Spencer, 2015). Since the specific experience of the aura is largely determined by the locus of seizure onset and seizure freedom requires complete removal or disconnection of the epileptogenic zone (EZ), it is important to recognize pitfalls in aura semiology and understand aura networks to improve seizure focus localization. Although multiple diagnostic modalities are available, the precise formulation of seizure semiology continues to be a daunting clinical challenge and an important source of surgical failure (Lesser et al., 1984; So, 2006; Carreno and Lüders, 2008).The EZ is conceptualized as a discrete hub of abnormal electrographic seizure activity, but in reality the clinical spectrum of an epileptic seizure often reflects more widespread network dysfunction. As seizures propagate along preferential neural pathways, they activate defined networks and generate clinical symptoms that can be either significant or non-markers of the EZ (Iannotti et al., 2016).
The epileptic aura typically occurs at seizure onset and therefore is regarded as particularly important anatomically and a singularly accurate marker of the EZ. However, this is not always the case as the EZ will remain undetected if an aura is preceded by seizure onset in a clinically silent brain region, or by too rapid electrical seizure propagation. Thus, an appropriate level of caution must be exercised before assigning definitive localizing value to any aura during the pre-surgical evaluation (Kellinghaus and Lüders, 2008; Tufenkjian and Lüders, 2012; Perven and So, 2015). These constraints underscore the importance of carefully correlating the clinical semiology of an aura through an understanding functional brain organization and the networks involved in epileptogenesis.
The localizing value and pitfalls of the epileptic aura
Visual and auditory auras
Visual auras include both simple and complex manifestations. In general, simple visual auras result from activation of primary visual cortex (Appel et al., 2015),while complex visual auras more likely reflect activation of association cortex in the temporo-occipital junction (Fried et al., 1995; Bien et al., 2000). In contrast, both simple and complex auditory auras remain confined to the lateral superior temporal lobe. Although the visual aura has important localizing power, complex visual auras often have widespread representation and can be elicited by activating basal as well as convexity temporal neocortex (Gupta et al., 1983). Complex visual hallucinations and visual illusions have also been described in seizures of frontal lobe seizure origin (Fornazzari et al., 1992; Beauvais et al., 2005; La Vega-Talbot et al., 2006; Ye et al., 2012),likely due to the extension of the ventral and dorsal visual stream into the prefrontal cortex (Wilson et al., 1993; O Scalaidhe et al., 1997; Ungerleider et al., 1998).
In contrast to visual sensations, auditory auras are decidedly less common (with an incidence of 1.3-1.9%) but also have variable localization (Penfield and Kristiansen, 1951). Simple unilateral auditory hallucinations including buzzing and ringing are well-localized to the temporal auditory cortex(Florindo et al., 2006) whereas complex hallucinations (music or voices) and positive illusions (increased loudness or altered frequency) reportedly arise in both the ipsilateral and contralateral cerebral hemispheres (Loddenkemper and Kotagal, 2005). Interestingly, negative illusions (auditory agnosia or hypoacusia) are associated exclusively with a dominant hemisphere EZ (Clarke et al., 2003, Florindo et al., 2006).
Vertiginous auras are often linked to visual or auditory symptoms (Richer et al., 1991) as they arise in the temporo-parietal junction in proximity to visual and auditory association areas. Direct cortical stimulation reveals extension of the vertiginous EZ above and below the Sylvian fissure, including the parietal operculum and the middle and posterior portions of the superior and middle temporal gyri (Kahane et al., 2003).
Olfactory and gustatory auras
Olfactory and gustatory auras are often linked together clinically making it difficult to differentiate them. The association of olfactory and gustatory auras is considered the defining feature of “uncinate fits”, but their localizing significance is not absolute. While olfactory auras are observed in seizures arising from the mesial temporal and occipital lobes (Palmini and Gloor, 1992; Acharya et al., 1998; Russo et al., 2016),olfactory sensations are also produced by stimulating the olfactory bulb, insular cortex, amygdala (attention to olfactory auras in case of amygdala neoplasm) and the posterior orbitofrontal region (Andy, 1967; Bancaud and Talairach, 1992).
Olfactory illusions hold special significance for tumor patients as they may arise in auditory association cortex and also propagate to connected areas including the insula and inferior frontal operculum (Isnard et al., 2004).
The risk of misidentification of the EZ is even greater for gustatory auras. Taken alone, the gustatory aura has little lateralizing or localizing value as stimulation of rolandic cortex, parietal operculum, insular cortex and mesiobasal temporal regions all generate gustatory hallucinations (Bancaud and Talairach, 1992; Russo et al., 2016). It has been proposed that gustatory hallucinations result from ictal spread to the operculum by “functional reorganization of connections within these epileptogenic areas” (Hausser-Hauw and Bancaud, 1987; Ostrowsky et al., 2000).
Somatosensory aura
Somatosensory auras have specific localizing and lateralizing value through their direct correlation with the somatosensory homunculus. Sensory auras occurring distally and unilaterally signify an EZ in primary sensory cortex, while bilateral and more widespread symptoms are often produced through activation of the second somatosensory area (Pugnaghi et al., 2011),whichalso generates ipsilateral sensory symptoms including feelings of pain or warmth (Stephani et al., 2011; Montavont et al., 2015).
Primary sensory symptoms (numbness and tingling) should be differentiated from complex symptoms including painful sensations (burning, pricking or muscle tearing), shivering and goosebumps. ECoG recordings of painful somatosensory auras localize the EZ to the posterior insula and secondary somatosensory cortex (Montavont et al., 2015), whereas autonomic auras including shivering and goosebumps originate predominantly from the dominant left temporal lobe (amygdala) (Stefan et al., 2002).Similarly, primary somatosensory auras of a supernumerary phantom limb (SPL) and missing limb arise from cortical areas outside primary sensory cortex (Millonig et al., 2011). SPL has also been reported from the temporo-parietal junction while a missing limb sensation arises from the right parietal region (Mauguiere and Courjon, 1978).
Focal somatic auras of pharyngeal or laryngeal dysesthesias usually indicate activation of posterior insular cortex with early spread to the temporal and parietal operculum (Isnard et al., 2004). These auras have rarely been reported to arise from mesial temporal cortex (Carmantet al., 1996) suggesting that these symptoms are produced through activation of insular-perisylvian networks with an ‘entry gate’ (insulo-operculum or operculum-insular) (Carmant et al., 1996).
Despite their high localizing value, somatosensory cortical stimulation studies reveal that somatosensory auras may overlap with symptoms arising in insular cortex and the supplementary sensorimotor area (Lim et al., 1994; Salanova et al., 1995; Beauvais et al., 2005).False interpretation of somatosensory auras may occur due to imperceptible muscle contractions after motor cortex stimulation (Bancaud et al., 1994). Reduced localizing and lateralizing value of sensations affecting the entire body has also been observed (Bartolomei et al., 2004). Somatosensory auras associated with temporal lobe epilepsy may result from secondary spread from the parietal lobe or posterior temporal region.
Psychic auras
Psychic auras are decidedly les common and most likely involve a complex network that connects the temporal neocortex (visual hallucinations) to limbic structures (dreamy state) (Gloor et al., 1982; Gloor, 1990). The psychic aura may also require theta band synchronization within mesial temporal structures participating in a widespread network involving primary visual and associated cortices (Barbeau et al., 2005; Vignal et al., 2007). Unlike the abovementioned networks, SEEG and SPECT studies suggest that ictal fear results from network imbalance involving the amygdala and the temporal pole that includes projections to orbitofrontal, anterior cingulate and medial frontal cortex (Gloor, 1990; Biraben et al., 2001; Bartolomei et al., 2004; Barbeau et al., 2005).
Autonomic aura
The urge to urinate, shortness of breath, sialorrhea, lacrimation, vomiting, retching, flushing, palpitation, goosebumps, generalized cold or warm feelings are different types of autonomic auras produced by activating not only the insular cortex and temporal lobe, but also the anterior cingulum, frontal lobe, supplementary sensorimotor area, postcentral parasagittal region and amygdala (Remillard et al., 1983; Calleja et al., 1988; Fish et al., 1993; Kotagal et al., 1995; Henkel et al., 2002). Inconclusive localizing value of autonomic auras is therefore to be expected. Network studies indicate that the amygdala and hippocampus are important hub regions and that secondary activation occurs preferentially to insular and medial frontal regions (Calleja et al., 1988).
Sexual auras
Sexual auras are erotic feelings and pleasurable genital sensations that are mediated predominantly in the temporal lobe (Remillard et al., 1983; Baird et al., 2007), whereas sexual automatisms constitute the motor components of sexual behavior that arise from orbitofrontal regions and should not be confused with sensory auras.
Sexual auras must also be differentiated from unpleasant superficial genital sensations that lack sexual content (Remillard et al., 1983). These sensations can be produced by stimulating primary somatosensory cortex at the parasagittal convexity, interhemispheric fissure or possibly the perisylvian region (Bancaud et al., 1970).
Multiple auras
Extra caution should be exercised in the interpretation of multiple auras, which were reported by 6% of 1,897 patients with epilepsy. These phenomena are purportedly associated with multifocal epilepsy, or activation of a neural network with access to more than one functional region (Nakken et al., 2009). Most importantly, multiple auras can either accompany different seizures or occur during the same seizure, either simultaneously or sequentially. Multiple auras may originate in a single EZ, usually in the insular or temporal lobe or in posterior quadrant foci, resulting from either sequential or simultaneous activation of multiple symptomatogenic zones. Lastly, they may also occur in multifocal epilepsy and must be differentiated from a focal seizure network with secondary propagation leading to different auras. Multiple auras may also represent distinctive sensory disturbances propagating along different pathways from a single focus (Gloor, 1990; Widdess-Walsh et al., 2007).
Focal seizures with preserved awareness versus auras
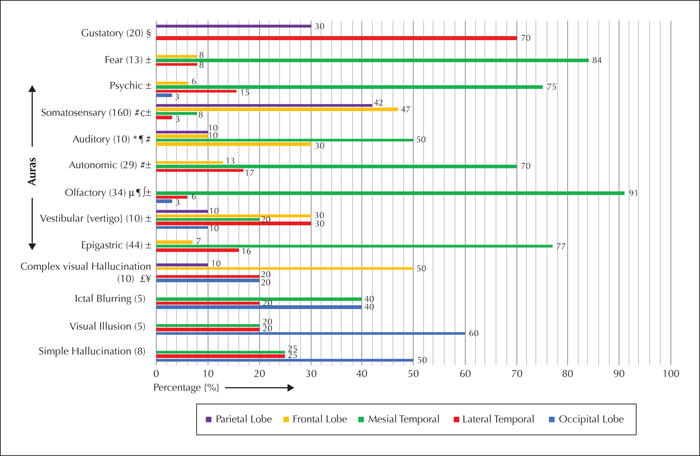
By definition, all focal seizures with preserved awareness are focal electro-clinical seizures that lack impaired awareness. Auras are a specific subset of focal seizures with preserved awareness characterized by a sensory state that reflects specific sensory alteration or altered perceptual awareness (e.g. distortion of sight, sound or smell, confusion, unpleasant emotions, dizziness or déjà vu feelings) (Ferrari-Marinho, 2012). An aura is therefore highly internalized and generates no observable change; it will only be discovered through careful history taking. In contrast, focal seizures with preserved awareness consisting of circumscribed motor phenomena are not strictly considered as auras as they generate outwardly observable manifestations that can be recorded by others (Taylor and Lochery, 1987). Auras provide an important warning that may prevent self-injury or injury to others. Most auras originate from posterior neocortex or deeper brain regions (mesial temporal, inferior frontal, etc.). As reviewed in figure 1, different brain regions that induce similar auras due to early spread will reduce their lateralizing and localizing value. Fortunately, focal seizures with preserved awareness most often present as focal seizures localized to one anatomical location.
Interictal experiential phenomena versus true epileptic auras
Experiential phenomena are complex psychic phenomena observed primarily in adults with temporal lobe or limbic epilepsy, and to differentiate them from the true epileptic auras would avoid localization errors. They are distinct from epileptic auras or focal-onset seizures arising in the posterior neocortex (Scheffer et al., 2017). Experiential phenomena create a compelling immediacy that is similar to or even more vivid than real life (Scheffer et al., 2017). They may present as illusions, complex (auditory-visual) hallucinations, fear or a déjà vu feeling; most are fragmented and lack detail (Gloor, 1990; Scheffer et al., 2017).Psychic phenomena can be elicited by direct cortical stimulation of the limbic structures - amygdala, hippocampus and parahippocampal gyrus and temporal neocortex (Gloor, 1990; Scheffer et al., 2017). PET brain imaging in patients with temporal lobe epilepsy and déjà vu reveals focal hypometabolism in parahippocampal regions suggesting a strong association with parahippocampal dysfunction (Guedj et al., 2010).Interestingly, no ictal after-discharges were noted during stimulation of these areas, suggesting that these experiences are positive interictal phenomena and neither an ictal event nor ictal paralysis (Guedj et al., 2010).Although psychic phenomena have important localizing potential, their localizing value in epilepsy surgery patients is limited as they are not true representations of ictal events or ictal onset (Chen et al., 2003). It is presumed that complex underlying networks are involved in eliciting experiential phenomena which usually begin as a limited discharge in a group of neurons that later utilizes strengthened connectivity to re-create a particular experience (Gloor, 1990). In children, experiential phenomena are poorly described, likely due to immaturity of long-term memory storage areas and the child's inability to describe complex feelings.
From the epileptogenic zone to the border territory: involved networks
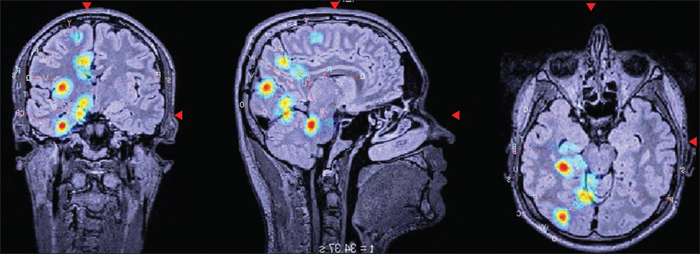
While a seizure onset zone may manifest as a simple visual or auditory aura, it is more often silent or triggered later in the propagation sequence (David et al., 2010).The dynamic interaction between the seizure onset zone and early involved brain areas, including their electrographic features will determine the semiology of the seizure, including the aura and its localizing value (Chauvel and McGonigal, 2014).By fully describing the entire sequence, from aura to postictal manifestations, the localization of the seizure onset zone becomes a probability analysis within a given network (Chauvel and McGonigal, 2014). SEEG studies may be required to ascertain a more precise analysis of the spatial-temporal propagation of the epileptic seizure in parallel with the semiological sequence (Bartolomei et al., 2017), by implementing the surgical outcome as shown in figure 2; the aura representing either part of the seizure onset zone or one of the first activated hubs in the epileptogenic network.
Electrical seizure onset typically (but not always) precedes the aura by seconds to minutes, although many patients do not experience auras during SEEG monitoring (David et al., 2010; Perven and So, 2015). This observation further emphasizes the importance of the aura, if experienced, as part of the seizure onset zone or one of the primary nodes in the propagation sequence in the epileptic seizure.
Psychic manifestations, such as fear or anxiety, are especially challenging to localize anatomically as fear often occurs as a non-specific sensation without an accompanying sensory disturbance. Biraben et al. (2001) studied the appearance of early fear in frontal and temporal lobe epilepsy.In two cases of right mesial temporal epilepsy, SEEG recordings identified the electrographic seizure onset in the amygdala 30 to 60 seconds before fear manifested, and concluded that a more widespread network that includes the frontal lobe is required for the expression of fear (Biraben et al., 2001). In four SEEG patients, the aura of fear appeared when electrical seizures propagated to external temporal lobe structures, the orbitofrontal cortex and anterior cingulate.
Auras arising from the frontal lobe are highly variable but their description may provide precise localization of seizure onset. Bonini et al. (2014) studied frontal lobe seizures in 54 selected patients, based on clustering their auras with their ictal semiology, and delineated four distinct networks (fronto-parietal, fronto-insular, widespread frontal and fronto-temporal). The authors observed a general tendency for seizures arising from premotor or prefrontal areas to propagate posteriorly, but there was no reverse propagation. The latency between electrographic and clinical seizure onset (either aura or other clinical signs) averaged three seconds (range: 0.5 to 10 seconds). Latencies differed between groups, with the shortest interval occurring in the group without aura who had seizures arising from premotor cortex. Somesthetic localized auras characterized the first group, with manifestations when the electrical seizure reached Broca Area 4. Auras with feelings of fear, anxiety or rage defined the fourth group, when the electrical seizure reached the Broca Areas 14, 24r, 32, amygdala and temporal structures. The second group expressed a non-localized aura, which mostly manifested when the electrical discharge involved the pre-SMA and the Broca Area 14.
Temporal lobe auras that progress to motor convulsions are particularly challenging. Ferri et al. (2014) utilized SEEG exploration to examine the role of the auditory aura in nocturnal frontal lobe epilepsy. They showed that seizure freedom occurred when the EZ was accurately localized to the temporal lobe. Propagation to the frontal lobe occurs rapidly in this disorder and the temporal EZ is easily overlooked if the aura is the only indicator of extra-frontal seizure origin. The authors concluded that the origins of the unilateral auditory auras were Heschl's gyrus and the temporal operculum, while no clear origin of the bilateral auditory aura was identified.
Specific temporal lobe auras provide important diagnostic value for seizures with rapid secondary spread.Barba et al. (2007) compared the scalp-recorded features of temporal and temporal-plus epilepsy.The type of aura was an important differentiating factor - abdominal auras were associated with purely temporal lobe seizures, while gustatory hallucinations, rotatory vertigo and auditory illusions were primarily associated with temporal-plus seizures. The origin of the abdominal aura, especially when followed in the seizure sequence by oral and/or manual automatisms, is considered to be mesial temporal structures, as previously described (Taylor and Lochery, 1987; Henkel et al., 2002; Barba et al., 2007). Gustatory aura is considered to arise from the opercular cortex and insula (Hauser-Hauw and Bancaud, 1987; Ostrowsky et al., 2000; Isnard et al., 2004; Barba et al., 2007) in seizures beginning in the temporal lobe and insular cortex. Rotatory or vestibular auras are believed to originate from the lateral temporo-parietal area and are the most important manifestation when the seizure onset zone is in the TPO junction (Kahane et al., 2003; Barba et al., 2007).
In contrast to auras of temporal lobe origin, parietal lobe auras are poorly localizing. Recently, Bartolomei et al. (2017) differentiated clinical from electrical parietal seizure networks.They identified four main networks, with some auras assisting in network definition. For example, vestibular auras localized to the superior parietal lobule, whereas somatosensory auras in the contralateral limbs were strongly indicative of parietal network involvement. Fear and anxiety were reported by two parietal lobe patients, reinforcing the anatomically non-specific basis of these symptoms.
A small number of patients will describe multiple auras (6% according to Nakken et al. [2009]), either in a sequence during the same seizure, or in different seizures. Insular epilepsy provides the clearest example of multiple auras occurring in a sequence, as insular epilepsy patients frequently describe a throat sensation followed by paresthesia in a delimited territory and elementary auditory hallucinations (Isnard, 2009), according to the hubs in the propagation sequence of the epileptic discharges to the parietal and temporal structures.In a study of 31 patients with multiple auras evaluated in the Cleveland Clinic, most with at least three aura types had a seizure onset zone in the non-dominant hemisphere, particularly the temporal lobe (Widdess-Walsh et al., 2007), a finding confirmed by others (Perven and So, 2015). The authors postulated that the experience of multiple auras strongly suggests localized epileptogenesis as consciousness is fully preserved. In some patients, secondary propagation to the contralateral hemisphere may occur, which will impair consciousness and block recall of the aura (Widdess-Walsh et al., 2007; Perven and So, 2015).
Multiple seizure types with multiple auras are rare indicators of multifocal epilepsy. Invasive recording may be required to distinguish this unique subgroup from patients with unifocal seizure onset and secondary activation sites (Widdess-Walsh et al., 2007).This issue is best studied by direct electrical stimulation utilizing depth or grid electrodes. Protocols using 1-Hz or 50-Hz stimulation can be utilized to define the EZ. Single-pulse electrical stimulation permits the study of aura-generating cortical networks to determine specific patterns and comparisons with normal brain networks (Perven and So, 2015; Matsumoto et al., 2017).
Auras in generalized epilepsy
Although epileptic auras are typically regarded as indicators of focal seizure onset, they are also described by patients with generalized absence and myoclonic epilepsy, and may occur in a similar proportion (21-70%) to focale epilepsies (Boylan et al., 2006; Gungor-Tuncer et al., 2012; Dugan et al., 2014). Common auras in patients with generalized epilepsies include visual auras (flashing lights, macropsia, illusional movement of the environment, blurred vision and ictal blindness), autonomic and dyscognitive symptoms (lapsed awareness, change in speech and thoughts or comprehension), hiccups, epigastric sensation, fear, unusual taste and unexplained changes in emotional state. The appearance of an aura in generalized epilepsy could represent selective focal cortical activation within a generalized network.Some pre-ictal symptoms or auras in generalized epilepsies are caused by repetitive spike-wave discharges prior to motor seizure onset. An accurate electro-anatomo-clinical correlation of aura symptomatology in generalized epilepsy would therefore be required to understand their precise localization (Boylan et al., 2006; Gungor-Tuncer et al., 2012; Dugan et al., 2014).
Developmental considerations
Maturation of seizure semiology progresses throughout the pre-adolescent years in parallel to the acquisition of cognitive and behavioral milestones (Fogarasi et al., 2007; Nordli, 2013).Auras are also influenced by maturational factors but are additionally dependent on the child's ability to communicate and relate to previous memories (Working Group of the International Association of the Scientific Study of Intellectual Disability, 2001). Until full language competence is achieved, aura description is unlikely (Fogarasi et al., 2007). Although auras have been described as early as three years of age, descriptions are more reliable after age six years (Fogarasi et al., 2007; Nordli, 2013). Until then, children can only recognize seizure onset if instructed. Their restricted world experience and limited feelings and sensations can yield erroneous aura information (Fontana et al., 2006).
These issues also occur in adults with intellectual deficiency (Verellen and Cavazos, 2011). Auras in the elderly population represent an additional challenge, as multiple comorbidities, minor daily complaints, and cognitive decline may obscure aura manifestations (Verellen and Cavazos, 2011).Because of these issues, a significant number of elderly patients remain undiagnosed with epilepsy for more than a year after seizure onset (DeToledo, 1999; Verellen and Cavazos, 2011).Language impairment or dementia also precludes meaningful description of their aura symptoms (Silveira et al., 2011).Auras in the elderly are thus rarely reported and poorly described; they are also frequently associated with dizziness (Verellen and Cavazos, 2011). Seizures in the elderly may manifest as confusional episodes or prolonged periods of postictal confusion lasting for weeks that make aura description nearly impossible (Cloyd et al., 2006; Silveira et al., 2011; Verellen and Cavazos, 2011; Stefan et al., 2014).
Disclosures
None of the authors have any conflict of interest to declare.