Epileptic Disorders
MENUMeasurable outcomes for pediatric epileptic encephalopathy: a single-center experience with corticosteroid therapy Volume 23, numéro 1, February 2021

Figure 1
Study subjects.
*Excluded due to (1) insufficient baseline clinical data (n=5); (2) insufficient follow-up clinical data (n=4), or (3) concomitant use of other immunomodulatory therapy (n=1). †Excluded from sub-analysis studies of dexamethasone vs. methylprednisolone due to no available follow-up long-term video-EEG (n=3) or the details of the steroid course were markedly different from that of other children in the cohort due to length or dosage (n=2).
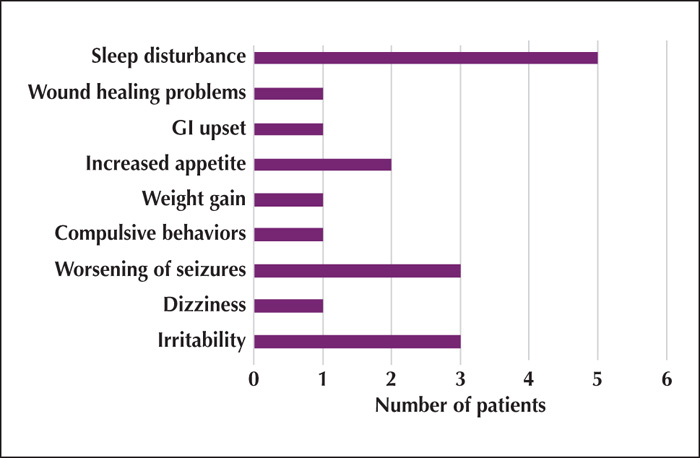
Figure 2
Example video EEG segments showing pre-treatment and post-steroid treatment EEG#1 (1 day post-treatment); sensitivity: 15 mV/mm; time base: 30 mm/sec.

