European Journal of Dermatology
MENUSafety and efficacy of secukinumab in psoriasis patients infected with hepatitis B virus: a retrospective study Volume 32, numéro 3, May-June 2022
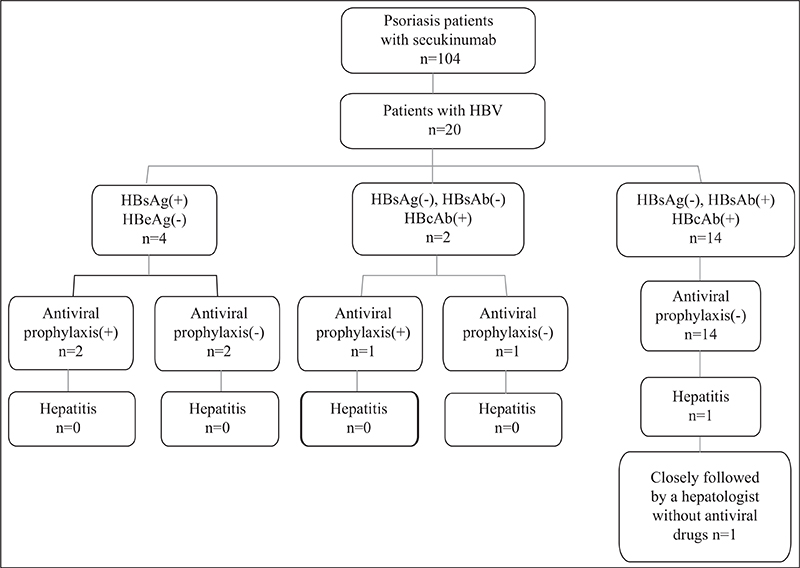
Figure 1.
Psoriasis patients with HBV infection on secukinumab therapy (schematic). HBV: hepatitis B virus; HBsAg: hepatitis B surface antigen; HBeAg: hepatitis B core e antigen; HBsAb: hepatitis B surface antibody; HBcAb: hepatitis B core antibody.
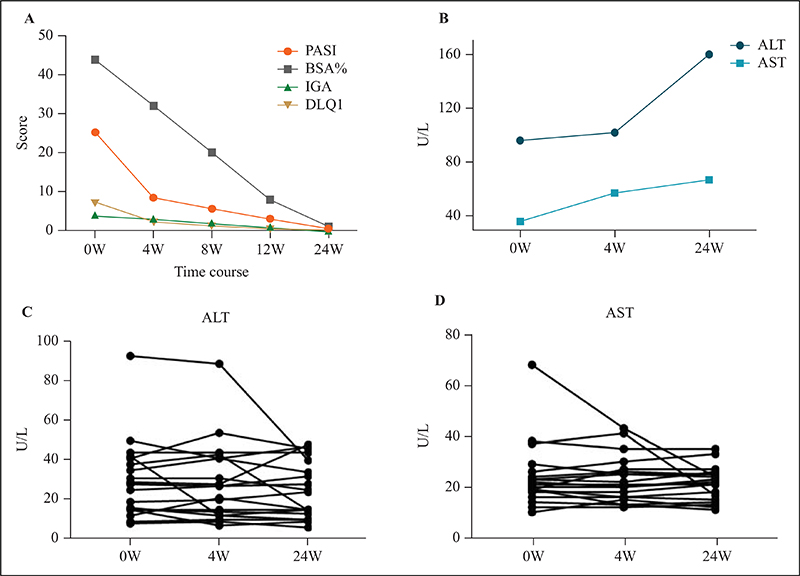
Figure 2.
Clinical course of one patient showing the Psoriasis Area Severity Index (PASI) score (orange line), the percent of body surface area (BSA) affected (grey line), Investigator Global Assessment (IGA) (green line), and Dermatology Life Quality Index (DLQI) score (yellow line) (A), as well as ALT level (upper limit of normal: 49 U/L) (dark-blue line) and AST level (upper limit of normal: 34 U/L) (light-blue line) (B). C) ALT level of the other 19 patients. D) AST level of the other 19 patients. ALT: alanine transaminase; AST: aspartate transaminase.
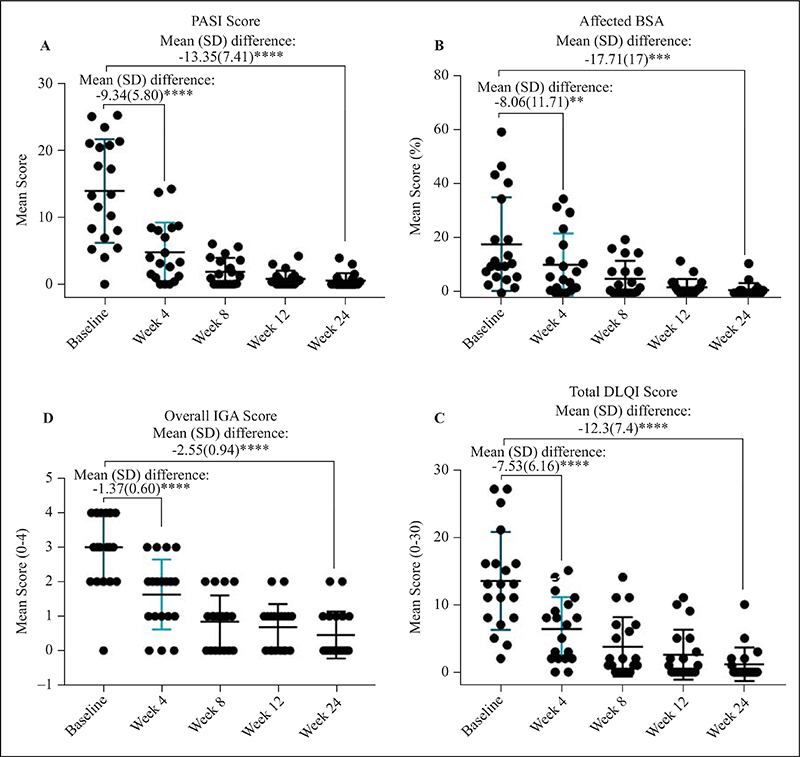
Figure 3.
PASI (A), % BSA affected (B), overall IGA score (C), and total DLQI score (D) at Weeks 0,4, 8, 12 and 24 in psoriasis patients with HBV infection on secukinumab therapy (**p <0.001, ****p <0.0001).
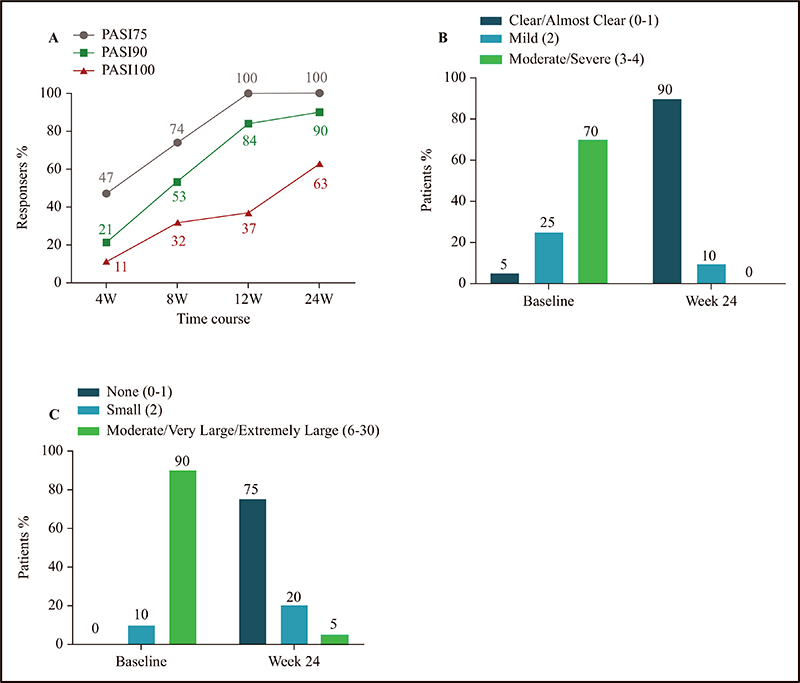
Figure 4.
A) The proportion of patients with PASI 75, PASI 90 and PASI 100 at 4, 8, 12 and 24 weeks. B) The proportion of patients in different IGA categories: clear/almost clear (0–1) (IGA score = 0–1); mild (2) (IGA score = 2); and moderate/severe (3–4) (IGA score = 3–4). C) The proportion of patients in different DLQI categories: none (0–1) (DLQI score = 0–1); small (2–5) (DLQI score = 2–5); and moderate/very large/extremely large (6–30) (DLQI score = 6–30). PASI 75: 75% reduction of PASI score; PASI 90: 90% reduction of PASI score; PASI 100: 100% reduction of PASI score.

