European Journal of Dermatology
MENUEfficacy and safety of clindamycin phosphate 1.2%/tretinoin 0.025% formulation for the treatment of acne vulgaris: pooled analysis of data from three randomised, double-blind, parallel-group, phase III studies Volume 24, numéro 2, March-April 2014
University of Nantes, Place Alexis Ricordeau, 44093, Nantes, France
Section of Dermatology Azienda Ospedaliera Universitaria of Ferrara,
Corso Giovecca 203, 44100 Ferrara, Italy
60590 Frankfurt/Main, Germany
Harrogate & District NHS Foundation Trust,
Lancaster Park Road, Harrogate, HG2 7SX, UK
Benzstrasse 1, 61353 Bad Homburg, Germany
Otto-von-Guericke University of Magdeburg, Universitätsplatz 2, 39106 Magdeburg, Germany
- Mots-clés : acne vulgaris, clindamycin phosphate, combination therapy, pooled analysis, tretinoin
- DOI : 10.1684/ejd.2014.2293
- Page(s) : 201-9
- Année de parution : 2014
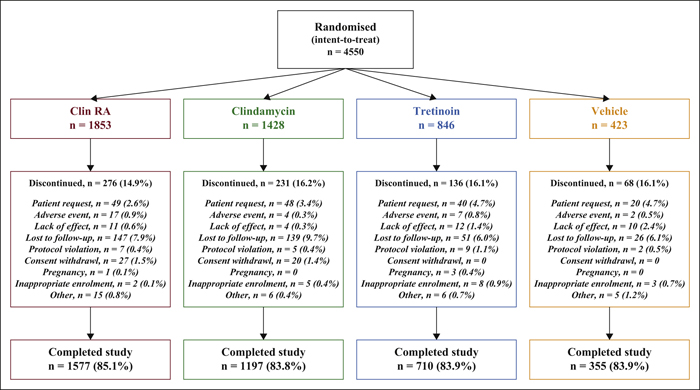
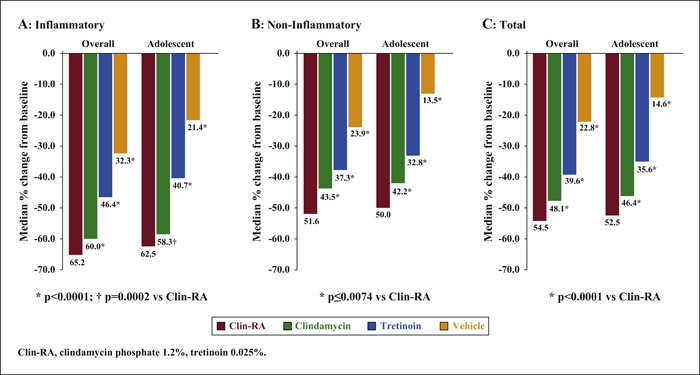
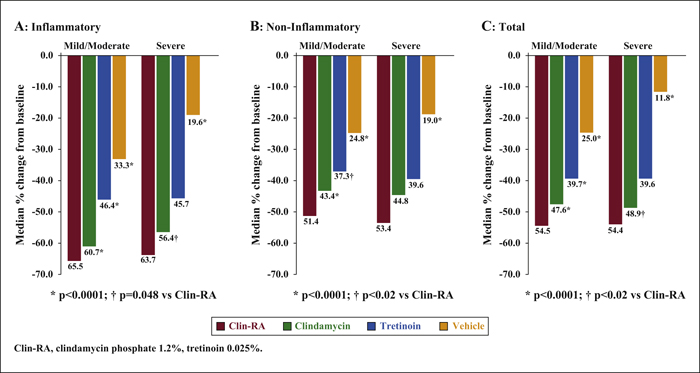
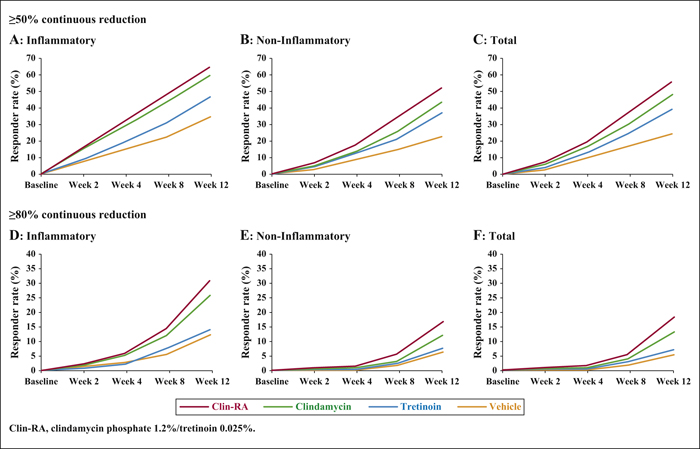
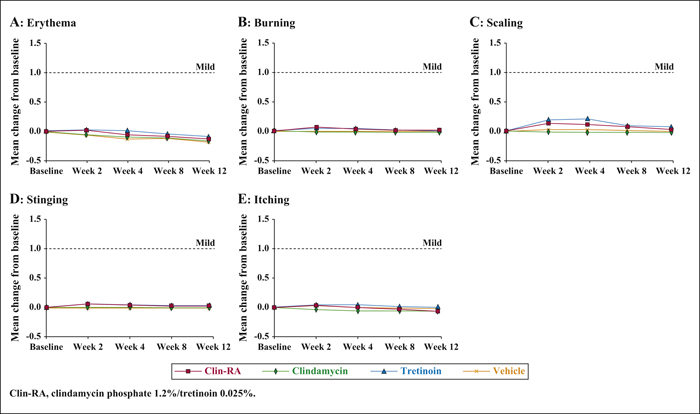
Background:The efficacy and safety of clindamycin phosphate 1.2%/tretinoin 0.025% (Clin-RA) were evaluated in three 12-week randomised studies. Objectives:To perform a pooled analysis of data from these studies to evaluate Clin-RA's efficacy and safety in a larger overall population, in subgroups of adolescents and according to acne severity. Materials & Methods: 4550 patients were randomised to Clin-RA, clindamycin, tretinoin and vehicle. Evaluations included percentage change in lesions, treatment success rate, proportions of patients with ≥50% or ≥80% continuous reduction in lesions, adverse events and cutaneous tolerability. Results: In the overall population, the percentage reduction in inflammatory, non-inflammatory and total lesions and the treatment success rate were significantly greater with Clin-RA compared with clindamycin, tretinoin and vehicle alone (all p<0.01). The percentage reduction in all types of lesions was also significantly greater with Clin-RA in the adolescent subgroup (2915 patients, p<0.002) and in patients with mild/moderate acne (3662 patients, p<0.02) versus comparators. In patients with severe acne (n = 880), the percentage reduction in all lesion types was significantly greater with Clin-RA versus vehicle (p<0.0001). A greater proportion of Clin-RA treated patients had a ≥50% or ≥80% continuous reduction in all types of lesions at week 12 compared with clindamycin, tretinoin and vehicle. Adverse event frequencies in the active and vehicle groups were similar. Baseline-adjusted mean tolerability scores over time were <1 (mild) and similar in all groups. Conclusion:Clin-RA is safe, has superior efficacy to its component monotherapies and should be considered as one of the first-line therapies for mild-to-moderate facial acne.