Annales de Biologie Clinique
MENUDifficile examen direct par coloration de Gram : à propos d’un cas d’endocardite à Cardiobacterium hominis et revue de la littérature Volume 77, numéro 5, Septembre-Octobre 2019
Cardiobacterium hominis is a Gram-negative rod described for the first time by Slotnick and Dougherty in 1964 after observation of some cases of endocarditis caused by a Pasteurella-like organism [1]. This respiratory tract's commensal bacterium, strictly human, belongs to the HACCEK group. These bacteria are responsible for rare cases of endocarditis infections (EI) [2], associated with a 3% of in-hospital mortality [3]. Long time incubation of blood-culture and slow-growing nature make C. hominis infections hard to diagnose and treat. We reported here a case of EI due to C. hominis, as well as a review of the literature on C. hominis infections (table 1), identification techniques and drug treatments.
Case report
A 59-years-old woman with a past medical history of Laubry-Pezzi syndrome (septal defect and aortic insufficiency), appendectomy, uterine fibroids and ovarian cysts was admitted to our hospital in a context of acute leukemia suspicion. The patient reported a fever, bruises and multiple pains for a few days. Complete blood count performed in a medical laboratory had revealed the presence of blast cells. She was then admitted to the hematology ward and type 4 acute myeloid leukemia was diagnosed.
At the admission, clinical examination revealed 39̊C fever, a weight of 52 kg for 1.5 m high, no nodal, splenic or cutaneous tumor syndrome. Echocardiography found a cardiac murmur due to her ventricular septal defect. Laboratory assessments showed white cells count as follows: 60.2 G/L with 2.4 G/L neutrophils, 7.2 G/L lymphocytes, 27.6 G/L dystrophic monocytes and 23.0 G/L blast cells. Hemoglobin rate was 8.2 g/dL and platelets count were 26.0 G/L.
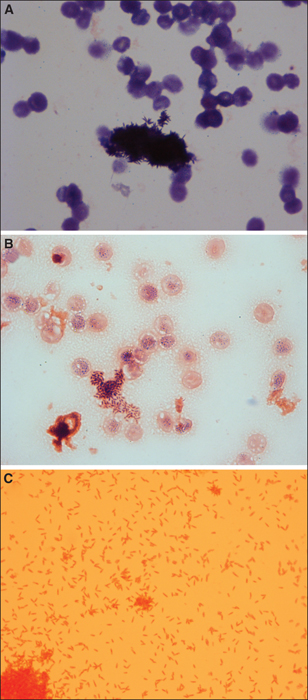
Two days after her admission, chemotherapy was started, including cytarabine and daunorubicin. Aplasia was reached in a few days. Because of persistence of fever and biological inflammatory syndrome with 147 mg/L CRP, intravenous antimicrobial drugs were started with piperacillin/tazobactam 4,000 mg/500 mg every eight hours and vancomycin 30 mg/kg/day. At this time, white cells were at 0.8 G/L with 0.2 G/L neutrophils. Meanwhile, peripheral aerobic blood culture was reported positive in 2 days and 23 hours to Gram-positive rods with a very unusual grouping mode in stack like sea urchin (figure 1A). Rods remained somehow Gram-positive in other area of the smear despite pink-colored red blood cells (figure 1B). After 72 hours, subcultures on blood agar revealed grey, round, smooth, opaque and glistening colonies with a size about 3 to 8 mm. Conversely to the first Gram-staining obtained from the culture bottle, the ones obtained from the colony showed Gram-negative rods in rosette suggesting C. hominis (figure 1B).Identification was performed using MALDI-TOF-MS (Microflex mass spectrometer, database MBT IVD Library -6763, Biotyper® 2.3, Brucker Daltonics, Bremen, Germany) and identified C. hominis with a 1,48 score despite complete extraction by formic acid and acetonitrile. Regarding this insufficient score, identification was confirmed using a phenotypic method using Vitek2 XL (bioMérieux, Marcy l’Etoile, France). The biochemical characteristics identified C. hominis with 85% of probability. For a complete characterization of the strain regarding this case report, we performed sequencing of the 5’ end of the 16S rRNA gene using 515F and 1492R primers [4], confirming C. hominis identification (accession number M35014), based on a 100% identity, using the BIBI and the NCBI database (figure 2). Interestingly, peripheral anaerobic blood culture of this series remained sterile after 5 days of incubation.
The next day, other blood culture samples (peripheral anaerobic and aerobic catheter blood cultures) turned positive to Gram-positive rods in rosette suggesting, once again, C. hominis. Delays of positivity were to 71h35 and 74h50, respectively. C. hominis identification was then confirmed using MALDI-TOF-MS also with a low score as mentioned above.
The minimal inhibitory concentration (MIC) of this C. hominis, determined using E-test method (bioMérieux, Marcy l’Etoile, France) on Muller Hinton agar were: amikacin (MIC = 0,25 mg/L), piperacillin/tazobactam (MIC < 0,016 mg/L), gentamicin (MIC = 0,064 mg/L) and cefepime (MIC = 0,064 mg/L). β-lactamase chromogenic assay was negative.
Transthoracic echography (TTE) showed a 65% left ventricular ejection fraction and no evidence of vegetation. Nonetheless, IE diagnosis was strongly considered given the very high link between C. hominis and IE and the past medical history of ventricular septal defect of the patient. Cerebral computer tomography (CT) did not show embolic lesions but hardly characterizable liver injurie were found on the thoraco-abdominopelvic CT. Due to the identification of the microorganism and the aplasia, treatment was switched to discontinuous intravenous piperacillin/tazobactam 4,000 mg/500 mg every eight hours and daily injection of gentamicin 8 mg/kg/day.
Two weeks later, 103 CFU/mL of AmpC overproducing-Citrobacter freundii was isolated in urine sample and in blood culture, leading to the change of the antimicrobial treatment for intravenous cefepime 2 g/j and amikacin 30 mg/kg/day. The patient was in remission and the aplasia ended three days later. Unfortunately, one week later, she showed respiratory distress and was transferred to the intensive care unit. She died one day later of severe sepsis associated with a multi-system organ failure, without any clue of the source of infection and without any microbiological documentation.
Discussion
C. hominis is an opportunistic bacterium belonging to the normal microbiota of oral and upper respiratory tract of human. Two species of Cardiobacterium can currently be described: C. hominis and C. valvarum. They belong to the HACCEK group, responsible for bacteremia and EI, and composed of Haemophilus spp (H. parainfluenzae), Aggregatibacter spp (A. aphrophilus, A. actinomycetemcomitans and A. segnis), [formerly Actinobacillus actinomycetemcomitans, Haemophilus aphrophilus and Haemophilus segnis until 2006[5], Cardiobacterium spp, Capnocytophaga spp (C. ochracea, C. sputigena and C. gingivalis for the human species), Eikenella corrodens and Kingella spp (K. kingae and K. denitrificans). A monocentric study of 45 cases of HACCEK endocarditis managed in the Mayo clinic (Minnesota, USA) between years 1970-1992 described that the HACCEK group was responsible for 1-3% of EI, with a proportion of 13-27% due to C. hominis and only 1% due to C. valvarum [6]. C. hominis is responsible for insidious EI, mainly in patients with congenital heart disease or prosthetic valves. In such cases, the disease is characterized by prolonged sub-acute courses associated with fever, splenomegaly, heart failure, anorexia and malaise. Relation between C. hominis bacteremia and EI is up to 95% which explains why EI was diagnosed in the presence of only one major and two minor criteria of IE [7, 8]. Ventricular septal defect, presented by our patient, corresponds to a failure of the inter-ventricular septum and is a congenital heart defect driving to an increased volume load, excessive pulmonary blood flow, reduced systemic cardiac outputs and high pulmonary artery pressure. Presence of a heart murmur is frequent with this pathology and can mask some endocarditis symptoms. Ventricular septal defects and others cardiac malformations have been described as predisposing causes of C. hominis EI [9].
C. hominis is a pleomorphic Gram-negative rod presenting a characteristic mode of grouping like rosettes [1]. This case report highlights the difficulty of the biological identification of C. hominis due to the variability of the Gram coloration. Culture of these bacteria is usually performed in standard enriched media and optimal growth is obtained with presence of 5% CO2. Culture is weak in microaerophilic atmosphere and negative in anaerobic atmosphere. Classically, 3.3 days are required to obtain a positive blood culture without treatment, but in some cases, culture could be extended until 14 days [9, 10]. C. hominis can be identified and differentiated from the other HACCEK bacteria by its biochemical characteristics which are fermentation of glucose, maltose, mannose, sucrose, mannitol, sorbitol and the production of indole [10]. However, these characteristics do not allow to differentiate C. hominis to C. valvarum. The only phenotypical difference described in the literature is the production of raffinose by C. hominis [11]. Differentiation between the two Cardiobacterium species can be made by broad-range 16S rRNA PCR/sequencing test on a culture colony or directly from the sample as described by Gatselis et al.[12]. Further studies used 16S rRNA PCR/sequencing to identify C. hominis, but with the arrival of MALDI-TOF-MS, 16S rRNA PCR/sequencing is now mainly used at distance of the infection or in case of negative culture. Today, detection of HACCEK bacteria and Cardiobacterium spp has been largely facilitated by the introduction of MALDI-TOF-MS, allowing a rapid and reliable identification, in particular between the two species of Cardiobacterium spp, with one spectrum of C. hominis and one of C. valvarum present in the database (MBT IVD Library - 6763). However, as described in the case report, a weak score of MALDI-TOF-MS may require a confirmation using complementary techniques. One of the main difficulties when determining antibiotic susceptibility of the HACCEK bacteria is based on the lack of clinical breakpoints by the European committee on antimicrobial susceptibility testing. Typically, HACCEK group bacteria are susceptible to β-lactams. Ampicillin and ampicillin/clavulanic acid were therefore generally used to treat HACCEK infection. However, a rapid increase of β-lactamase producing-strains led to a modification of the guidelines [13]. Nowadays, third generation cephalosporins for 4 weeks are the first line to treat IE due to the HACCEK group [8]. Fluoroquinolones can be used as an alternative and aminopenicillin are now reserved for the non β-lactamase producing-strains. Conversely to the other HACCEK group bacteria, β-lactamase producing-C. hominis are still very rare and only few cases of β-lactams resistance have been reported in literature [13, 14].
Conclusion
The present case illustrates the difficulties and the diagnostic approach allowing to identify C.hominis, a Gram-negative rod mainly responsible for EI. Prognosis of Cardiobactrium spp EI is generally favorable, but the delayed diagnosis caused by the late identification of the bacteria can have severe consequences, particularly at cardiac level. Despite emergence of few antimicrobial resistance determinant, Cardiobacterium spp remains susceptible to the third generation cephalosporins which provides a quickly and efficient therapy. The arrival of the MALDI-TOF-MS has greatly facilitated the diagnosis of this infection, but bacterial phenotypical and biological characteristics, as well as the 16S rRNA PCR may still be used to confirm the identification in case of low score.
Conflict of interest
none of the authors has any conflict of interest to disclose.
Acknowledgements
All the authors would like to thank Imogen Fersen for her review of the manuscript.
![]() Cette œuvre est mise à disposition selon les termes de la
Licence Creative Commons Attribution - Pas d'Utilisation Commerciale - Pas de Modification 4.0 International
Cette œuvre est mise à disposition selon les termes de la
Licence Creative Commons Attribution - Pas d'Utilisation Commerciale - Pas de Modification 4.0 International