Hépato-Gastro & Oncologie Digestive
MENUPrevention of chemotherapy-induced neutropenia in digestive oncology Volume 26, supplement 1, Octobre 2019
- Key words: neutropenia, febrile neutropenia, chemotherapy, gastrointestinal cancers, G-CSF
- DOI : 10.1684/hpg.2019.1832
- Page(s) : 27-33
- Published in: 2019
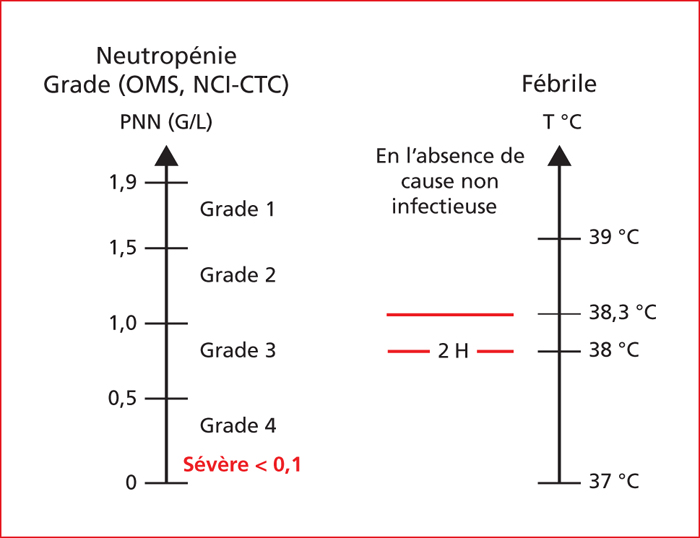
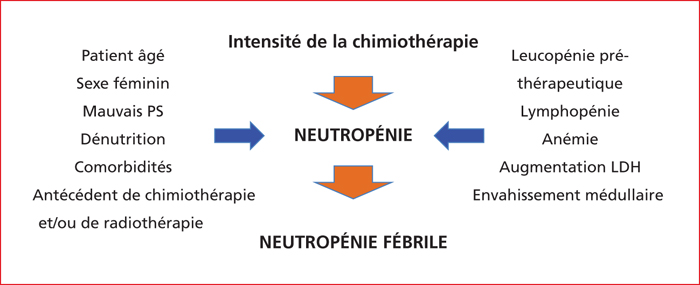
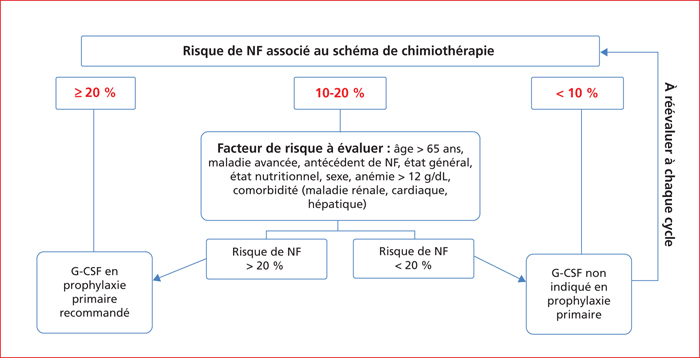
The risk of severe chemotherapy-induced neutropenia varies according to the chemotherapy regimen and clinico-biological factors. For instance, concerning the chemotherapy-based treatment of colorectal cancer, the risks of severe neutropenia that is grade 3 to 4 (<1.000 neutrophils/mm3) and febrile neutropenia range from 2% to 50 % and <1% to 10 %, respectively. For the majority of chemotherapy regimens prescribed in gastrointestinal oncology, the prevention and management of severe chemotherapy-induced neutropenia is mainly based on delaying cycles generally associated with decreasing drug dosage to the detriment of the regimen's intensity-dose ratio. For regimens associated with a higher risk of severe neutropenia such as DCF, VP16-platinum and FOLFIRINOX regimens, the prescription of G-CSF in primary or secondary prophylaxis should be discussed or recommended systematically. The search for a DPD deficiency before the prescription of 5-FU helps to prevent the risk of severe haematological toxicity related to 5-FU. The management of the risk of neutropenia attributable to the prescription of chemotherapy requires a good knowledge of the tolerance profiles of the prescribed chemotherapies and risk factors related to the patient.
![]() This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License