Hématologie
MENUNew molecular classifications of diffuse large-cell lymphomas Ahead of print
Diffuse large B-cell lymphomas (DLBCLs) are the most common (30-40%) non-Hodgkin's lymphomas (NHL) in Western countries. More than half of these lymphomas can be cured by approaches combining monoclonal antibodies (anti-CD20) and chemotherapy, most often including anthracyclines. Around 30-40% of these lymphomas, however, prove to be refractory or relapse, suggesting significant underlying biological heterogeneity. The seminal work published by Alizadeh et al., almost 20 years ago, using DNA chips for the first time in oncology, identified two major subtypes of DLBCL based on the expression of genes reflecting the presumed cell of origin (COO) of the tumour cell: subtype GCB (for germinal centre B-cell-like), referring to a centroblastic origin; and subtype ABC (for activated B-cell-like), referring to cells that have reached post-germinal centre (GC) maturation [1]. The GCB and ABC subtypes were recognised in the 2016 World Health Organization (WHO) classification and the 2017 revised classification (table 1), which recommends that they are identified using molecular biology techniques or, failing that, immunohistochemistry [2, 3].
Following this publication, several studies clarified the prognostic value of these subtypes and the biological differences that characterise them, such as the prevalence of MYC, BCL2 and BCL6 rearrangements and their respective protein expression, mutation profiles, copy number variants (CNV), and associated signalling and B cell receptors (BCRs).
Although this dichotomy has largely dominated the debate concerning the heterogeneity of DLBCL, it remains paradoxically almost inoperative in clinical practice today, and has a very marginal or non-existent impact on treatment strategies. In addition, 10-20% of DLBCLs remain unclassified according to this model which, therefore, only partially reflects the complexity of DLBCLs. New technologies, mainly next-generation sequencing (NGS) and integrated bioinformatic analysis, have now made it possible to establish new molecular classifications that more accurately describe the biological bedrock of DLBCLs. Another major development proposed by the 2016 WHO classification is the individualisation of high-grade B-cell lymphomas (HGBLs), characterised by an aggressive clinical presentation, an unfavourable prognosis, and the presence of a translocation involving MYC and BCL2 and/or BCL6. Once again, recent work has clarified the molecular outline of this subtype. This article reviews these new classifications and their potential clinical impact (figure 1).
Questioning the clinical relevance of the GCB/ABC dichotomy
Due to the lack of robustness and reproducibility of immunohistochemical (IHC) techniques, molecular techniques leading to gene expression profiles (GEP) are currently recommended for the diagnosis of GCB/ABC phenotypes. However, relatively little work has been undertaken on the molecular identification of GCB/ABC subtypes in large cohorts of patients based on prospective trials.
In a study by the German High-Grade Lymphoma Study Group (GHGLSG), the GCB/ABC profile was established using the Lymph2Cx chip (developed by Nanostring), based on the differential expression of around 20 genes [4]. Rearrangements of MYC/BCL2/BCL6 were identified by fluorescent in situ hybridisation (FISH). Protein expression of MYC (+40% threshold) and BCL2 (+50% threshold) was also established to identify “double-expressor” (DE) patients. Subjects with first-line DLBCL were included in the RICOVER60 trial (> 60 years of age, with any international prognostic index (IPI), randomised between R-CHOP21 and R-CHOP141), and the R-MegaCHOEP trial (n = 88) and therefore insufficient to detect a difference, unlike the RICOVER cohort (n = 326). On the other hand, the authors showed that DE patients are a poor prognostic group, but only for the GCB subgroup, as the prognostic value is lost in the ABC subtype.
Given the distinct biological characteristics of the GCB and ABC subtypes, several trials have investigated the value of combining R-CHOP with drug X which specifically targets one of the metabolic pathways associated with a subtype.
Two prospective randomised trials reported disappointing results regarding the predictive (theranostic) value of the GCB/ABC phenotype. The prospective Phase III REMoDL-B multicentre trial evaluated the value of the combination of R-CHOP + bortezomib (RB-CHOP). First of all, this trial showed that “real-time” molecular biological identification of GCB/ABC status is possible using FFPE [5]. Of the 1,128 patients in the trial, 918 (81%) were effectively classified according to their COO: 244 (27%) were ABC, 475 (52%) were GCB, and 199 (22%) were unclassified. After the first cycle of R-CHOP, phenotyped patients were then randomised and received either R-CHOP or RB-CHOP. The trial concluded that the addition of bortezomib does not provide any benefit in terms of PFS, either for the whole population (PFS at 30 months: 70.1% [95% CI: 65.0-74.7] with R-CHOP versus 74.3% [69.3–78.7] with RB-CHOP; HR = 0.84; 95% CI: 0.64-1.11; p = 0.23), or for molecular subgroups, in particular ABCs. However, Davis et al. highlighted the potential benefit of the combination for patients with double-hit or DE lymphoma, although this benefit is not significant in terms of PFS or OS (see below). In summary, these results indicate that, despite the overexpression of the NF-κB pathway in ABC DLBCLs, the addition of bortezomib does not provide any advantage over R-CHOP. Several reasons can be put forward to explain these disappointing results: the relative under-representation of the ABC subtype, older ABC patients, the use of bortezomib only from the second cycle onwards and at relatively low doses and, finally, the distinction between GCB/ABC that is probably too simplistic to identify patients likely to benefit from such a combination [5, 6].
The objective of the Phoenix (NCT01855750) randomised, double-blind Phase III trial was to improve the efficacy of R-CHOP in combination with ibrutinib in patients with non-GCB or ABC DLBCL [7]. Eight hundred and thirty-eight non-GCB patients were included based on Hans’ immunohistochemical criteria. The primary objective, as measured by EFS, was not met, showing a similar EFS with intent-to-treat for both treatment groups. For the 567 patients classified as ABC, neither was of any benefit according to molecular biology. However, perhaps encouragingly, based a pre-specified analysis of the subgroup of patients under 60 years of age, the R-CHOP + ibrutinib regimen showed benefits in terms of both PFS and OS (HR: 0.330, (95% CI: 0.162–0.673). Indeed, the toxicity of this combination in patients over 60 years of age is probably partly responsible for the overall negative conclusion of the trial, since this led to a significant reduction in the dose intensity of R-CHOP. Analysis of the mutational profiles of patients likely to benefit from such a combination is ongoing in this cohort.
Finally, the results of the ROBUST trial (Phase III randomised trial, NCT02285062), which were presented at the 2019 American Society of Clinical Oncology meeting, were also negative, showing that the addition of lenalidomide to R-CHOP (R2CHOP) for ABC-subtype DLBCLs compared to a placebo was of no interest.
More encouragingly, data from the CAVALLI Phase II trial (NCT02055820) suggest, with regard to the GOYA cohort, greater efficacy of R-CHOP combined with ventoclax, a BH3 (for BCL2 homology 3) mimetic, in BCL2-positive patients (50% IHC threshold), regardless of molecular subtype.
These three negative trials raise the question of the choice of molecule and biomarker (GCB/ABC) used and suggest that a single biomarker (expression of BCL2 quantified by IHC) may be more relevant than COO for patient selection and improvement of R-CHOP outcomes.
A thousand and one exomes
Reddy et al. reported the analysis of more than 1,000 cases of DLBCL (1,001 to be exact!) using whole-exome sequencing (WES), RNA sequencing (RNAseq), SNP (single nucleotide polymorphism) arrays, and gene expression profiles (GEP, Nanostring Technologies) [8]. The molecular landscape is described very precisely with this impressive number of cases, complementing data that has already been published on more than 500 WES cases. It should be noted, however, that only 502 samples in this study could be compared with their constitutional counterparts.
Some concepts have been confirmed: for example, 20 genes are differentially mutated according to their phenotype, including EZH2, SGK1, GNA13, SOCS1, STAT6, and TNFRSF14 for GCB, and ETV6, MYD88, PIM1, or TBL1XR1 for ABC. On the other hand, 150 recurrently mutated genes have been identified and considered as drivers, some of which have been rarely reported until now (e.g. BTK, SPEN, CDKN2A, RB1 and CD70). On average, DLCBLs are targeted by 7.75 mutations in these drivergenes. Their driving role is demonstrated by genomic editingapproaches such as CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats associated with protein9) using six cell lines (three ABCs, two GCBs and one Burkitt line). Thus, genes essential for cell growth in the majority of cell lines are identified and considered to be oncogenic: MYC, RHOA, SF3B1, MTOR or BCL2. This approach also allows the identification of genes that behave as tumour suppressors: TP53, MGA, PTEN, NFKBIE, RB1 or NCOR1. Interestingly, of the driver genes thus identified, nine are specific for a molecular subtype: the inactivation of EBF1, IRF4, CARD11, MYD88 or IKBKB are thus lethal for ABC lines while the inactivation of ZBTB7A, XPO1, TGFBR2 and PTPN6 genes are lethal for GCB lines, offering distinct therapeutic opportunities.
Concerning the expression data obtained by RNAseq, 31 sets of genes were ultimately identified, defining relatively homogeneous molecular subgroups. Of these subgroups, the one defined by RHOA alterations is of particular interest, as these have been validated in vivo. Indeed, these are correlated with a “proliferation” type signature and a worse prognosis. Inactivation of RHOA by CRISPR-Cas9 is lethal to GCB and ABC cells. RHO knock-out mice have a significantly reduced B-cell count at the expense of T-cells, and there is a change in germ centre architecture, actin filament alterations and cell migration [35].
Finally, the authors propose a relatively complex prognostic model including genetic alterations of the 150 drivergenes, MYC/BCL2 expression, MYC rearrangements and the GCB/ABC phenotype. In this model, the most pejorative subgroup is represented by rearranged or mutated MYC or MYC-over-expressing patients. At the opposite end of the spectrum, mutated CD70 DLBCLs with GCB phenotype are considered to be lower risk lymphomas. By defining three strata, this prognostic model appears to outperform the COO phenotype, the expression of MYC and BCL2 or the IPI.
This study marks a turning point in the molecular analysis of DLBCL by integrating genomic, transcriptomic, and functional analyses. However, its clinical significance is still uncertain, including a prognostic model that appears to be difficult to transpose into routine practice.
New molecular subgroups
In a report published in the New England Journal of Medicine, Schmitz et al. proposed the basis for a new molecular classification by integrating next-generation sequencing (NGS) and PEG data from a cohort of 574 DLBCLs [9]. The cohort was purposely and significantly enriched in ABC or unclassified subtypes (Tables 2, 3). Based on an exhaustive genomic analysis, the authors proposed a classification into four homogeneous subtypes defined as follows:
- –MCD: based on the co-occurrence of MYD88 and CD79B mutations,
- –BN2: based on the high frequencyof BCL6 fusions and NOTCH2 mutations,
- –N1: based on the presence of NOTCH1 mutations,
- –EZB: based on the presence of EZH2 mutations and BCL2 translocations.
These subtypes are defined by distinct prognoses under R-CHOP, with a more favourable prognosis for subtypes BN2 and EZB than for MCD and N1, independent of IPI.
Despite the considerable volume of data reported in this study, several limitations should be noted. This classification only concerns 45% of DLBCLs, leaving the vast majority unclassified. As such, the choice to expand the collection with sub-ABCs and unclassified DLBCLs is not an accurate representation of the “real life” of DLBCLs observed in current practice. Interestingly, TET2 gene abnormalities were reported to be the most common genetic abnormalities (more than 10%) in this unclassified group (55%), suggesting that other approaches and cohorts are needed in order to better characterise this group. In the absence of a FISH analysis, the authors also failed to take MYC rearrangements into account in their classification.
The clinical and prognostic data of the Schimtz et al. classification are also preliminary and merit confirmation. This concerns a subgroup of 240 patients treated with R-CHOP with sometimes very small numbers for certain subtypes. Some biomarkers such as MYD88 mutations, reported in this study as having a poor prognostic impact, have been the subject of contradictory results in the literature [10-12].
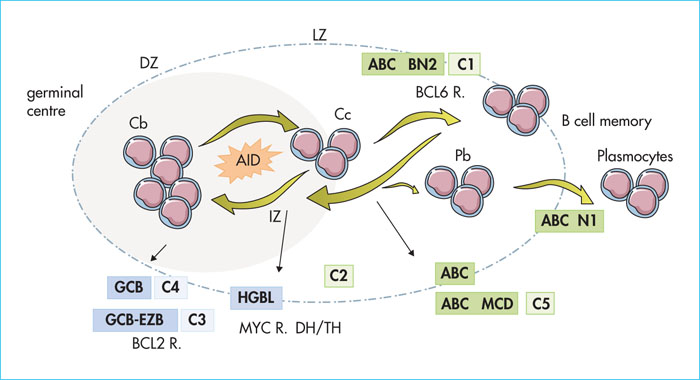
At the same time, the Shipp group proposed another model, based on 304 DLBCLs, which has some similarities with the model described above (tables 2, 3) [13, 14]. This uses mutation and CNV data to establish a classification divided into five clusters, from C1 to C5 (a C0 group without recurrent and minor abnormalities is also isolated, corresponding, in part, to T-cell-rich B lymphomas) [13]:
- –C1 = ABC, favourable prognosis, NOTCH2 mutations,
- –C2 = unrelated to GCB/ABC subtypes, inactivation of TP53 and CDKN2A, genetic instability,
- –C3 = GCB, poor prognosis, translocations of BCL2, alterations in PTEN and genes involved in epigenetics (MLL2, CREBBP, EZH2),
- –C4 = GCB, favourable prognosis, genetic abnormalities targeting BCR/PI3K, NFKB, RAS-JAK/STAT (BRAF, STAT3) or histones,
- –C5 = ABC, poor prognosis, BCL2 gain-of-function, MYD88 (L265P)/CD79B/PIM1/PRDM1 mutations.
High-grade B lymphomas
In addition to DLBCLs, defined based on their morphology and expression profiles (GCB/ABC), the WHO classification identifies the HGBL subgroup. These are defined based on morphological criteria (Burkitt-like morphology in particular) or on the presence of a rearrangement of MYC and BCL2 or BCL6, known as double-hits/triple-hits (DH/TH). These HGBL-DH/TH are of poor prognostic value and may require specific treatment. In the majority of cases, these lymphomas express a GCB profile. Two teams have recently identified a signature specific to these lymphomas [15, 16]. From a cohort of 157 GCB DLBCLs, including 25 rearranged HGBL-DH/TH BCL2, a signature (double-hit signature or DHIT) of 104 genes was defined. This DHIT signature was identified in 27% DLBCL-GCBs and was associated with an unfavourable prognosis regardless of DH/TH status since it could be present in the absence of these translocations. A distinct mutational profile is also identified in comparison with other GCB lymphomas without a DHIT signature. For example, mutations more often target the MYC, DDX3X, CREBBP, BCL2, EZH2 (Y646), TP53 and MLL2 genes [15, 17]. For routine clinical applications, this signature of 194 genes could be reduced for analysis of the expression of 30 genes, identified by Nanostring and validated in an independent cohort (DLBCL90 assay). These lymphomas are CD10+/MUM1- and have an expression profile corresponding to cells in transit/intermediate between the dark zone (DZ) and light zone (LZ), known as the intermediate zone (IZ), which has recently been defined, suggesting that HGBLs are derived from these cells [18]. The DHIT signature is characterised in particular by the overexpression of MYC and E2F genes, mTORC1, or those involved in oxidative phosphorylation. Inversely, HGBLs have low HLA I-II expression and low CD4+ T-cell infiltration. Interestingly, all the DLBCL-GCB lymphoma lines tested express this DHIT profile. Similar work was carried out on a cohort of 928 patients included in the RemoDEl-B trial (see above). The authors used a previously defined Burkitt signature (high-grade molecular signature, HGM) and applied it to this cohort [19]. Eighty-three patients (9% of the cohort) were thus isolated. Slightly fewer than half hadan MYC rearrangement (48.6%) or were DH/TH (36.1%). The expression profile was characterised by a proliferative signature and a “centroblastic” profile. This signature was only observed in GCBs and, interestingly, DLBCL-DH-GCBs without an MHG signature had a favourable prognosis and were identical to the other GCBs. The MHG signature is closer to DLBCL-GCB than to Burkitt lymphoma (BL). However, MHGs and BLs share an overexpression of MYC, genes involved in the cell cycle, TCF3 targets and ribosomal genes, as well as a centroblast (DZ) expression profile and high expression of FOXP1. The addition of bortezomib may improve the prognosis of MHG. As in the previously mentioned studies, a low level of expression of HLA molecules and immune response genes is observed in the MHG subtype. The MHG mutation profile is characteristic of DLBCL-GCB and also includes MYC mutations. Mutations in TCF3 and ID3, on the other hand, are less frequent than in BLs, suggesting distinct molecular mechanisms between the two entities. Although there is a good correlation between MYC/BCL2 expression at the mRNA level and protein level based on IHC, the MHG group is associated with a poor prognostic group, regardless of DE status [16]. This work suggests the importance of a precise molecular definition of HGBL, going beyond DH/TH lymphomas defined by FISH.
Conclusion
Twenty years after its definition, the concept of separating the GCB and ABC subtypes is still inoperative for clinicians. Therapeutic trials aimed at improving the efficacy of standard R-CHOP by targeting more specific alterations of the ABC subtype have so far all been negative, thus bringing the relevance of this dichotomy for patient stratification into question. New classifications integrating molecular data are proposed and enrich our understanding of the heterogeneity of DLBCLs. However, can these new, complex classifications, which incorporate technologies that are not easily accessible, be routinely applied in order to stratify patients? How can FISH data be integrated for MYC – this technique was recommended by WHO in 2016 to identify double-hits or HGBL [20]. What will be the real impact of these classifications on therapeutic strategies? These are all issues that will require further prospective and retrospective analysis. In addition to molecular classifications, other approaches are emerging that may be more relevant to guide treatment. For example, recent work shows that the expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a glycolytic enzyme, is predictive of the response to R-CHOP [21]. By controlling the pathways of glutamine metabolism, mTOR1, oxidative phosphorylation or mitochondrial metabolism, GAPDH proves to be a biomarker of the metabolic heterogeneity of DLBCLs, which is poorly defined by these molecular classifications, independent of COO, BCL2 or MYC expression.
EZH2 and MYD88, two driver genes in diffuse large B-cell lymphomas
EZH2 (enhancer of zeste 2 polycomb repressive complex 2 subunit) belongs to the transcriptional repressor complex, PRC2. Its catalytic domain allows the trimethylation of histone 3 on lysine 27 (H3K27m3). The protein is expressed in the B lymphocytes of the germ centre (GC) and is involved in its differentiation [22]. EZH2 can also monomethylate RORα (retinoic acid receptor related orphan receptor α), a protein induced by DNA alterations, promoting p53 activation and apoptosis. EZH2 and the PRC2 complex play a crucial role in GC formation by repressing genes involved in cell cycle control (such as CDKN1A) and altering the response to DNA damage [22]. EZH2 also represses genes involved in plasma cell differentiation such as IRF4 or PRDM1, thereby maintaining a GC-like phenotype. EZH2 has recently been shown to function in association with BCL6 protein (for B-cell lymphoma 6) by allowing the recruitment of another repressor complex, PRC1/BCOR complex (polycomb repressive complex 2/BCL6co-repressor), and CBX8 protein (chromobox 8). These PRC2 and PRC1/BCOR protein complexes bind, via CBX8, to “bivalent” promoters containing both repressor (H3K27me3) and activator (H3K4me3) states of chromatin.
The hot-spot mutations described in DLBCL and follicular lymphomas (LF) increase the catalytic activity of EZH2 and lead to greater repression of the target genes [23]. Mutant EZH2Y641mice develop GC hyperplasia and accumulate the chromatin repression marker, H3K27me3. The favourable prognostic value of EZH2 mutations has been highlighted by several studies in PMLs [24]. On the other hand, the favourable prognostic impact is uncertain in DLBCLs with contradictory results [8, 9]. Activating mutations appear to confer greater sensitivity to EZH2 inhibitors targeting its catalytic domain. Molecules currently in development also target other molecules in the PRC2 complex, including the embryonic ectoderm development (EED) cofactor [25].
MYD88 and the Toll-like receptor pathwayToll-like receptors (TLRs) are part of the PRR (pattern recognition receptor) family and are involved in innate immunity in response to many bacterial, viral, fungal, parasitic, or endogenous pathogens. TLRs are transmembrane proteins comprising a leucine-rich extracellular domain and a cytoplasmic domain homologous to that of interleukin 1. Ten LRTs are described in humans, making it possible to recognise a virtually unlimited number of pathogens. All except TLR3 depend on MYD88[26].
MYD88 mutations are found in ∼30% ABC-type DLBCLs, 70% primary cutaneous leg lymphomas, 44% intravascular lymphomas, and 38-100% primary lymphomas of the central nervous system or with testicular localisation [27-29]. They are most often heterozygous but may be associated with copy abnormalities (gain). In almost two thirds of cases, the L265P hot-spot affecting the TIR domain is observed in subtype ABC [30]. Alternative variants are described and are equally distributed in the GCB and ABC subtypes. In a mouse model reproducing the L265P mutation, the development of a lymphoma with the characteristics of ABC-type DLBCLs observed in humans can be seen [31]. The prognostic value of mutations is controversial and seems to be mainly related to the ABC phenotype and the older age of mutated patients. However, their presence may be associated with a greater risk of neuromeningeal relapse and extraganglionic localisation [30, 32, 33]. The hot-spot mutation can be detected in circulating DNA in plasma by digital PCR [34]. The TLR/MYD88 pathway can be pharmacologically targeted and is the subject of numerous preclinical investigations; IRAK4/IRAK1 inhibitors (interleukin-1 receptor-associated kinase 1/4) and molecules blocking the homodimerisation of MYD88 or heterodimerisation of TLR are currently being explored.
Conflicts of interest
The authors declare that they have no conflicts of interest affecting this article.
1 Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone in twenty-one or fourteen day cycles (R-CHOP21/14).