Epileptic Disorders
MENUElectroencephalography: basic biophysical and technological aspects important for clinical applications Volume 22, issue 6, December 2020
Figures
-
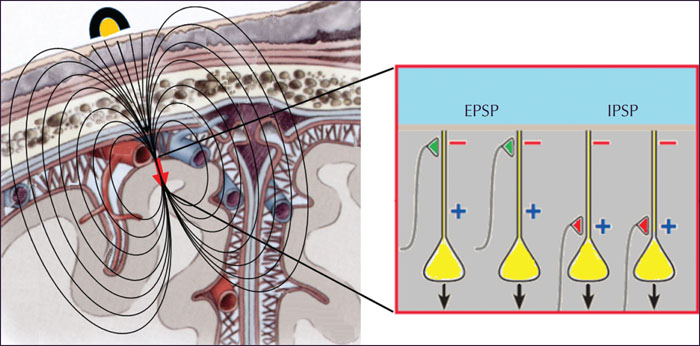
Figure 1 -
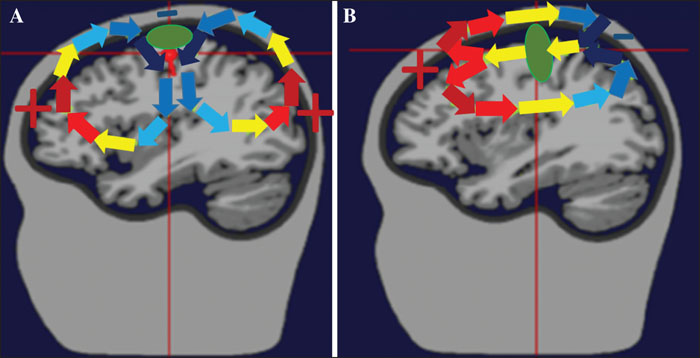
Figure 2 -
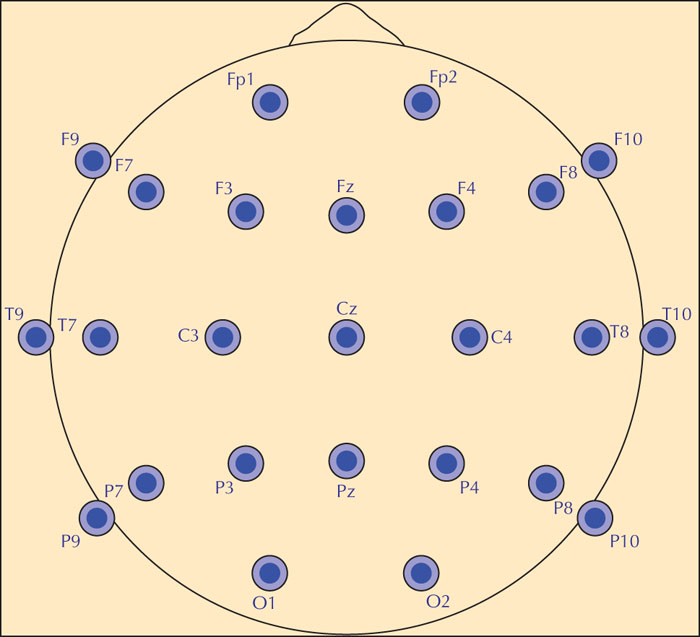
Figure 3 -
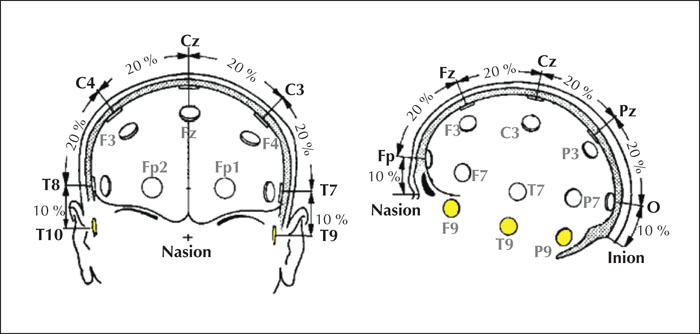
Figure 4 -
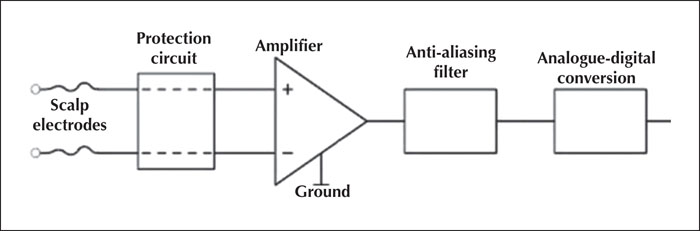
Figure 5 -
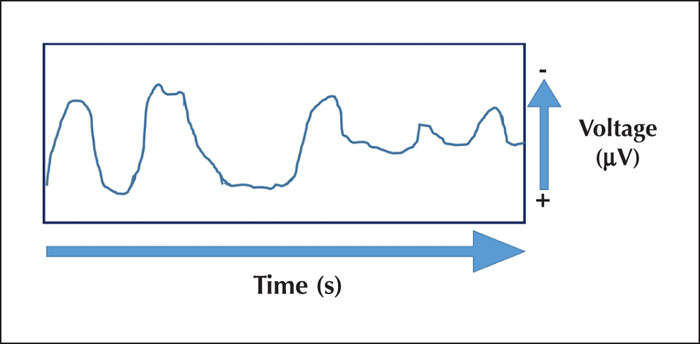
Figure 6 -
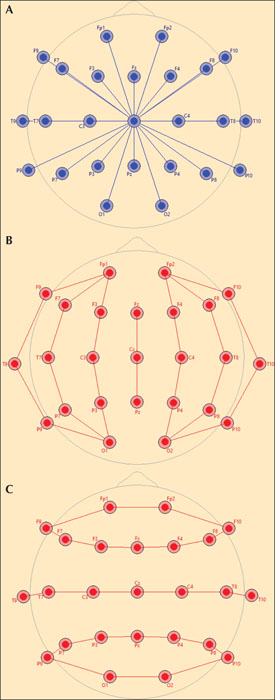
Figure 7 -
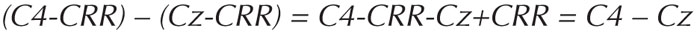
-
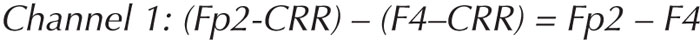
-
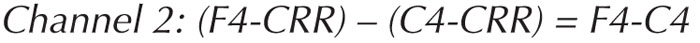
-
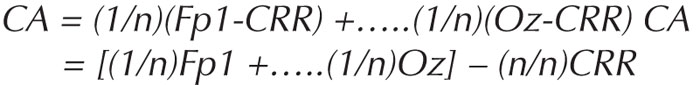
-
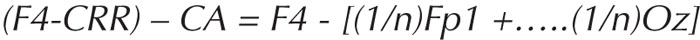
-
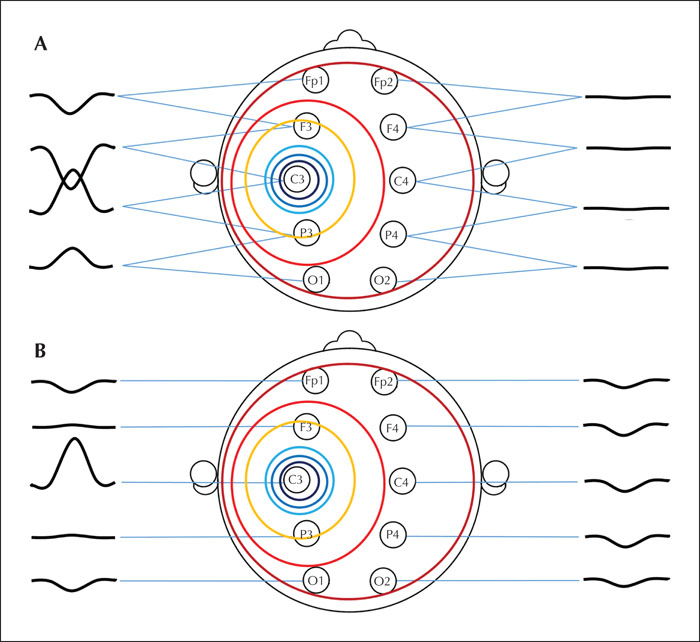
Figure 8 -
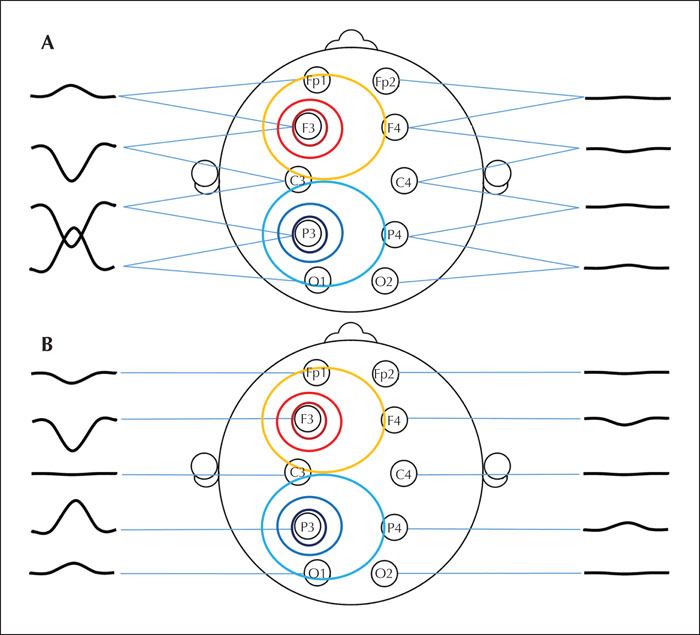
Figure 9 -
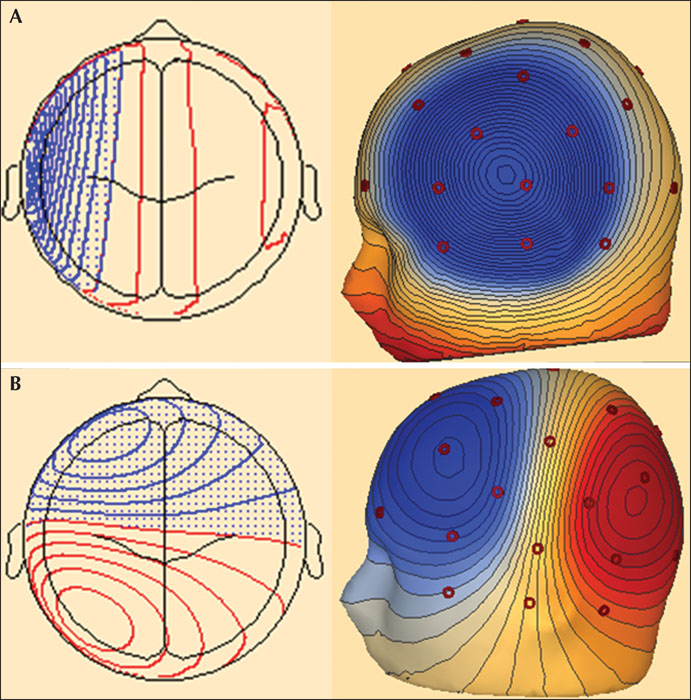
Figure 10 -
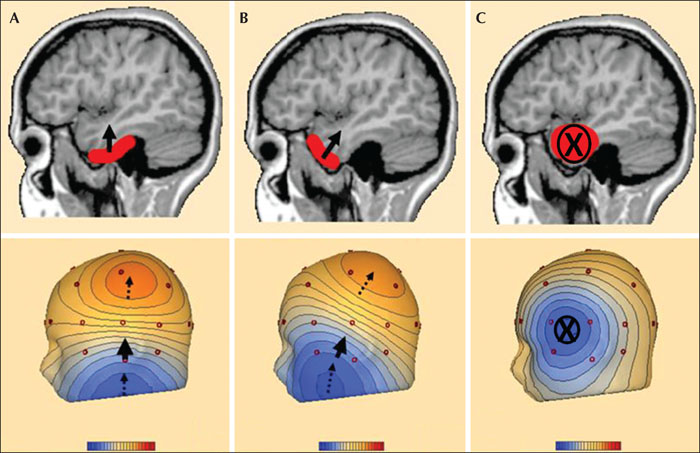
Figure 11
Tables
Electroencephalography (EEG) is the most often utilized tool in the diagnostic workup of patients suspected of having seizures or epilepsy (Tatum et al., 2018). The diagnostic significance of abnormal EEG patterns is well documented (Pillai and Sperling, 2006; Tatum et al., 2018). In skilled hands, EEG provides unique and vital information for many patients (Engel, 1984; Tatum et al., 2018).
It is essential that physicians managing patients with epilepsy understand the basic biophysical aspects of signal generation and recording technology in order to be able to interpret clinical EEG. This educational review paper explains how EEG signals are generated in the brain and how they are recorded / processed by the EEG machine. Special emphasis is given to learning objectives listed in the curriculum for epileptologists, recently issued by the International League against Epilepsy (Blümcke et al., 2019).
- –1.4.2 Demonstrate knowledge on how to conduct EEG recordings, including technical requirements;
- –1.4.3 Demonstrate knowledge of montages - advantages and disadvantages;
- –1.4.4 Interpret topographic (voltage) maps;
- –1.4.5 Recognize the indications for the different types of provocation methods;
- –1.4.6 Recognize the different types of EEG recordings and methodologies.
The electric source of the EEG signals
The electric signal recorded by EEG is generated by the ionic currents in the dendritic membrane of the pyramidal neurons in cortical layers IV-V (Amzica and Lopes da Silva, 2018). The cellular membrane is an electric insulator. Therefore, one cannot record intracellular currents using scalp electrodes. EEG records currents flowing in the extracellular space. These local field potentials are produced by these extracellular neuronal generators, excitatory and inhibitory postsynaptic potentials (EPSPs and IPSPs), and flow through the brain tissue and skull to the recording electrodes on the scalp; see explanation below. This biophysical phenomenon is called “volume-conduction”. Action potentials, on the other hand, do not contribute to the EEG signals. They are relatively random and are confined to a small number of neurons and are of short durations. Since they are relatively random and they generate very small and anatomically discrete activity; they are essentially lost in the background activity and contribute little to the signals recorded by scalp electrodes. However, less specific activation of the dendrites or somas that produce either excitation or inhibition can be synchronized to produce electrical potentials between the proximal and distal regions of a dendrite which produces an electrical dipole. The cell bodies of these neurons are in cortical layers IV and V and are arranged in “palisades” that are parallel to each other and perpendicular to the surface of the cortex (figure 1). Their dendrites extend to the more superficial cortical layers. Excitatory inputs are mainly into the distal regions of the dendrite while inhibitory inputs are closer to the cell body. This relative anatomical separation between excitation versus inhibition allows for the dipole to exist. When large numbers of neurons are synchronized, the net effect of the EPSPs or IPSPs is summated and can be seen by the scalp-based electrode.
For an EPSP, the sub-threshold depolarization of the dendritic membrane causes an influx of positive charges, mainly Na+, into the cell, leaving a negative extracellular charge where the synapse is located (active sink). Due to the compensatory return currents, a positive extracellular charge exists at a distance along the dendritic membrane (passive source). For the IPSP, the change in the extracellular electric charges is the opposite, i.e., a local, extracellular positivity at the synapse, due to the influx of negative charges (Cl-) into the cell (active source) and local, extracellular negativity at the passive sink, due to the compensatory current flow (figure 1). The generated extracellular current has a dipolar distribution along the apical dendrite, flowing from the source to the sink (figure 1).
To record an EEG pattern, such as the alpha rhythm or interictal epileptiform discharges (spike, sharp-wave, etc.) approximately 10 cm2 of cortex have to be activated, i.e., synchronized, simultaneously depending on the degree of synchronization (Tao et al., 2005). This limits the spatial resolution of EEG. The summation of the numerous, small dipoles (as described above) results in currents that are large enough to cross the tissues between the generator and the recording electrode. The dipoles generating interictal epileptiform discharges have a specific orientation, with the negative pole being closer to the superficial cortical layers (far from the cell body) and the positive pole being closer to the deeper layers (closer to the cell body). The most plausible explanation for this specific orientation is the different distribution along the apical dendrites of the excitatory and inhibitory synapses of the cortico-cortical connections that generate the interictal epileptiform discharges, as mentioned above, where the excitatory synapses are far from the cell body and the inhibitory synapses are close to the cell body (figure 1). The position and orientation of the cortical area generating the EEG signal determines the distribution of the negative and positive potentials on the scalp. This is the scalp topography, which can be visualized using voltage maps or amplitude-based maps. Figure 2 shows how volume conduction of the currents generated by different orientations (radial, tangential) of the cortical generators determine the scalp topography.
EEG electrodes and recording arrays
Electrodes make the contact between the patient and the EEG machine. Electrodes record the electric input of the EEG apparatus. In this paper, we address the non-invasive EEG recordings. Implanted electrodes such as subdural or depth electrodes will be described in detail in another seminar paper, on invasive EEG recordings.
Scalp electrodes are the most commonly used of all electrode types in EEG (Sinha et al., 2016). Scalp electrodes are made of non-polarized material, usually silver/silver chloride or gold. The contact surface is a cupped disc of up to 10 mm in diameter, filled with electrolytic paste. Thus, a steady-state ion flow exists between two layers of charge that have opposite polarity, i.e., the metallic surface of the electrode and the electrolytic paste. This generates the electrode potential. When a voltage is applied to the two layers, a current flows between the surrounding tissue and the electrode. This current is dependent on the ratio between voltage and resistance, as defined by Ohm's law where V = I X R (where V represents voltage, I is current, and R is resistance). Impedance reflects the combined effects of resistance and capacitance, and replaces resistance in alternating current circuits where V = I X Z (where Z represents impedance). Current-flow polarizes some metals, causing an increase in capacitance and changing the conduction properties. To avoid signal instability, non-polarized (inert) metals, such as silver-silver chloride or gold, must be used for EEG electrodes. The electrodes should not significantly attenuate signals between 0.5 and 70 Hz.
The electrode impedance depends on the electrical resistance of the skin and the contact between the skin and the electrolytic paste. An electrode impedance greater than 5,000 Ohms can cause noise artifacts. Since modern EEG equipment has high-input impedances, electrode impedances up to 10,000 Ohms are acceptable, provided they are balanced (Usakli, 2010). The electrode impedance should also not be below 100 Ohms because this usually indicates a “salt bridge” or a short between two neighboring electrodes on the scalp (Sinha et al., 2016). Skin-preparing agents must be used before the electrodes are applied, to remove dirt and oil from the skin and decrease the impedance. The conducting paste should have a high concentration of NaCl. A good contact between the electrode, conducting paste and skin must be maintained throughout the recording. Electrode impedance should be checked after applying the electrodes to the scalp, at the beginning of the recording and again at the end of the recording. If any doubt should arise during the recording, impedances can be checked again to reassure that the contacts are all in order.
Scalp electrodes are placed at standard positions (figure 3), according to the guideline of the International Federation of Clinical Neurophysiology (IFCN) (Seeck et al., 2017). The standard IFCN array is an extension of the so-called “international 10-20 system” (Sharbrough, 1997). It uses stable anatomical points of the skull such as nasion (point between the forehead and nose) and inion (bump at the back of skull) as well as the pre-auricular point (figure 4). Distances between the skull landmarks are measured (nasion to inion; left pre-auricular to right pre-auricular), and electrodes are spaced at 10% or 20% of the total distances between them (figure 4). The accompanying video in the supplementary material shows the electrode positions.
The standardized names of the electrode positions (figure 3) consist of two symbols. The first symbol is a letter abbreviation of the underlying brain region and the second is a number indicating its more precise position within that region. The abbreviations are Fp (frontal-polar), F (frontal), C (central sulcus), P (parietal), O (occipital), and T (temporal). Sagittal (midline) electrodes have the additional letter “z” instead of a number; Fz, Cz, and Pz. Even-numbered electrodes are located on the right side of the head, and odd-numbered electrodes on the left. Electrodes with lower numbers are closer to the midline and electrodes with higher numbers are farther away from the midline. F7 and F8 are close not only to the frontal cortex, as indicated by their names, but also to the pole of the temporal lobe. Thus, they record signals from the temporal lobe in addition to the frontal lobe. The geometric aspect is included as part of the electrode nomenclature, however, P7 and P8 have a misleading name, as they are situated over the posterior part of the temporal, and not the parietal region. Note that four electrodes are labelled differently in the old 10-20 nomenclature, but still used in many EEG laboratories (T7=T3; T8=T4; P7=T5; P8=T6). The standard IFCN array extends the classic 10-20 system with the electrodes in the inferior temporal chain, marked in yellow in figure 4. This has been necessary because the 10-20 system does not cover the inferior or basal part of the temporal lobe, and adding the inferior chain increases the diagnostic yield of interictal recordings and the localization accuracy of ictal recordings (Rosenzweig et al., 2014; Bach Justesen et al., 2018).
Sphenoidal electrodes, made of stainless steel, silver or platinum wires, with a small, bare recording tip, are inserted through a needle cannula under the zygomatic arch, so that the tip of the electrode is located laterally to the foramen ovale. Complications associated with their insertion include local discomfort, rare infections and the severing of branches of trigeminal and/or facial nerves. It was considered that the proximity of the sphenoidal electrode to the pole of the temporal lobe and the orbitofrontal areas would increase its diagnostic yield. All wire or needle-based electrodes should be used with great caution in patients with bleeding diatheses and are contraindicated in patients with known infectious disorders, especially those that involve the skin. Since the placement of these electrodes requires trained staff, there is the added risk of injury to staff personnel from the needle itself. However, these electrodes are still extra-cranial and the added value of the sphenoidal electrodes is controversial (Kanner et al., 2002). In our experience, the sphenoidal electrodes do not record more or better EEG signals compared to the scalp electrodes placed in the inferior temporal chain (see below). Yet, they cause discomfort to the patients and therefore we do not recommend using them. Subdermal needle or wire electrodes may be used for prolonged EEG recordings in comatose patients, in situations where application of cup electrodes is not feasible because of personnel or time constraints (Sinha et al., 2016). For these conditions, only disposable EEG electrodes should be used. All multi-use electrodes must be disinfected after each recording. Special disinfection procedures need to be adhered to when recording for patients with certain contagious diseases (Scott, 2013).
A denser electrode array can be achieved by placing all electrodes at a 10% distance or at an even higher density, at 5% distance (Seeck et al., 2017). Such high-density, evenly distributed arrays are useful for a more precise source localization that use mathematical models. The high-density electrode array is less feasible for long-term recordings, and the added value in standard (routine) recordings is not evident (Bach Justesen et al., 2019). With computational techniques (interpolation), the electric potential can be estimated even at points on the scalp between the real recording electrodes.
In addition to these scalp EEG electrodes, isolated ground electrodes should be placed and connected to the jackbox, as specified by the manufacturer. Most digital EEG recorders require one or more reference electrodes, placed on the scalp.
Besides the scalp EEG electrodes, a one-channel ECG channel should be added to all EEG recordings. In selected cases, electromyography (EMG) channels can be added for recording motor seizures, and respiration sensors can be added for recording changes in respiration rate, e.g., ictal apnea especially in the neonatal / pediatric setting. Similar logic holds for the use of a respiration monitor in neonates when assessing states of vigilance and in the intensive care units to aid in the determination of a variety of somewhat unique sources of artifacts. Additional electrodes around the horizontal and vertical axes of the eyes are helpful in staging sleep activity.
EEG amplifiers
The electric signals, recorded by the EEG electrodes are fed into the amplifier (figure 5), which contains many channels, one for each active electrode on the scalp. The protection circuit or isolation transformer ensures that the current only flows from the patient to the machine, not the other way around, thus protecting the patient from electric shocks that might originate from the EEG apparatus.
Each EEG channel measures the voltage, i.e. the difference in electric potential between the two electrode inputs, and the active and reference electrode which are fed into that channel's differential amplifier. The active electrode is one of the scalp electrodes, as described above. The reference electrode used during the recording in most of the modern digital EEG machines is an additional electrode placed on the scalp. This is called the “common recording reference” (CRR). For example, in the channel in which C3 is the active electrode, the voltage measured is between C3 and CRR; for P3 this is between P3 and CRR. Later, after analogue-digital conversion, these values are re-montaged.
The amplitudes of the signals from the brain, recorded by the scalp electrodes, are very small and in the order of μVs. The value of the electric potential at each scalp electrode is largely influenced by high-amplitude signals that are volume conducted to the scalp from other parts of the body. However, these large signals are present at each scalp electrode. Therefore, they cancel out when subtracting the potential at the CRR from the potential at the active electrode. This enables the EEG channels to reflect voltage originating from the underlying brain.
The analogue electric signals are digitally converted, i.e. changed into numbers or “virtual” signals in the computer. The voltage of the analogue electric signal in each channel is measured during very short, consecutive time windows or “time-frames” and the corresponding number is saved in the computer (digital signal). The voltage is then measured in the next time-frame, and so on. Each EEG channel thus shows the change in time of the voltage, such that the resolution in time is given by the duration of the “time-frame”. How short does this time-frame need to be for EEG purposes? To accurately represent a rhythmic signal in which a cycle demonstrates a change in positive and negative polarity, one needs at least two measurements during each cycle that are ideally at each polarity. For a signal of 40 Hz or 40 polarity changes in one second, one needs to measure the voltage (sample) at least 80 times per second or twice the number of cycles. This gives a sampling frequency of 80 samples per second or 80 “time-frames” during one second. Therefore, 1000 ms divided by 80 gives a “time-frame” of 12.5 msecond. The electric signals relevant for standard clinical interpretation are in the frequency range between 0.1 and 70 Hz. Because the sampling frequency has to be at least twice the value of the highest frequency required to be recorded, the minimum standard for sampling frequency for clinical EEG is 128 samples/second. However, for a better characterization of the signal, i.e., better resolution in time, a higher sampling frequency is desirable. Most of the modern EEG machines can record at 256 Hz and even higher. If one includes EMG electrodes into the recording array, that would impose additional needs. EMG signals include higher frequency domains, and therefore the sampling rates would need to be much higher (≥1 kHz).
Filters
If the sampling frequency is lower than half of the signal frequency, the recording is distorted. This phenomenon is called “aliasing”. EEG electrodes are able to pick up electrical noise at high frequency, e.g., from the muscles in the scalp, which results in the induction of artifacts, etc. To prevent distortion of the recordings due to these higher frequency components, signals are filtered using an analogue filter or anti-aliasing filter before analogue-to-digital conversion (figure 5). The effect of this filter is permanent.
After analogue-to-digital conversion, the digital signals can be further filtered, using selective digital filters. However, the effect of the digital filters is not permanent, since this is merely post-processing of the digital recordings. Many of the undesired signals are within frequency ranges that differ from those of EEG signals generated from the brain, and therefore can be removed or attenuated using digital filters when one is reviewing recordings. However, there are several important pitfalls when using digital filters. If one filters out a component within the range 0.1-70 Hz, then one can potentially lose relevant EEG data. Frequency components within the range of the filter values, and close to them, become distorted.
High-frequency or low-pass filters attenuate the components of frequency higher than the value of the filter. The filters attenuate the amplitude of signals at the cut-off value of the filter by 20-30%, and even higher frequencies are attenuated to a greater degree, even to complete elimination. Muscle artifacts, including surface-EMG signals from the muscles in the scalp, have higher frequency components than most EEG signals. However, filtering them out may also remove a high-frequency burst of EEG signals such as spikes. The other problem is that filtering out the muscle activity only attenuates these signals, and what is left may resemble a burst of spikes/sharp-waves and lead to erroneous interpretation.
Low-frequency or high-pass filters attenuate the slow components. The low-frequency range includes movement and sweating artifacts, which can be greatly attenuated by the use of these filters. The downside of too much low-frequency filtering is a loss or attenuation of actual focal or generalized pathological activity. In an extreme case, such as a recording of hypsarrhythmia, the EEG can almost be made to appear relatively normal. The influence of the low-frequency filter is determined by the time constant which is defined as the time required for the amplitude of a square wave to decrease to 37% of its original value (Sharbrough, 1997). The cut-off frequency can be calculated by dividing 0.16 (1/2π) by the numerical value of the time constant. For example, with a cut-off frequency of 0.16 Hz, the time constant is 1 second. Increasing the value of the low-frequency filter too much can remove some clinically important slow waves, or distort the form of the slow EEG pattern. An example would be the distortion of a blink artifact to such a degree that the correct interpretation becomes difficult.
Notch filters affect only a narrow frequency range and are primarily used to eliminate the electrical noise caused by power-line current; 60 Hz in North America and 50 Hz in Europe. It is important to begin all recordings with the notch filter deactivated so that the technician can be alerted to “bad electrodes” with weak contacts between electrode, paste, and scalp. When one starts to review an EEG recording, the recommended filter settings are 0.5 Hz to 1 Hz for the high-pass and 70 Hz for the low-pass digital filter, with the selective 60-Hz or 50-Hz filter turned off (Sinha et al., 2016).
Calibration
Calibration is performed to assess the accuracy of the EEG apparatus. A signal is generated within the EEG apparatus and is then recorded. The signal is then checked for several parameters by the machine itself as internal calibration. This consists of a known square-wave input voltage, ranging from 1 μV to 10 mV, which passes through the various phases of the EEG signal processing. The calibration is an integral part of every EEG recording (Sinha et al., 2016).
Display: visualization of the EEG signals
Voltage measured consecutively at each time-frame or time-window are displayed on the screen. Each EEG channel is similar to an oscilloscope in which time runs horizontally from left to right and voltage is presented on the vertical axis (figure 6). Thus, we visualize voltage changes over time. As most of the clinically relevant EEG patterns demonstrate mainly negative scalp polarity, by convention, negative potentials are upwards, and positive potentials are downwards (figure 6).
EEGs are usually reviewed by displaying 10 seconds of recording on the computer screen or 15-20 seconds on wide screens. One second corresponds to 30 mm on the horizontal axis of the oscilloscope. This is called “paper speed” because, on the old analogue EEG machines, the speed of the paper roll determined the resolution in time. In digital EEG, one can change the time resolution offline. Using a higher “paper speed” i.e., fewer seconds of recording displayed on the screen, enables one to visually inspect more details that are not so obvious at the standard “paper speed”. For example, the rapid propagation of a signal from one region of the brain to another may become visible. Another example is the relative ease of differentiating EEG signals versus EMG activity seen during seizures with motor manifestations. On the other hand, a lower “paper speed”, i.e. longer time-epoch displayed on the screen, makes one appreciate slow waves better than at the conventional paper speed. Lower paper speed is used frequently in polysomnography recording and for neonatal recordings in which identifying epochs of discontinuity is made easier. Also, slow periodic events are easier to identify using slower display speed.
The gain or sensitivity is the relationship between the magnitude of the input electrical potential in the channel and the corresponding measure of the deflection of the EEG trace on the screen reflected in the “size” of the signal displayed on the screen. It is expressed in μV/mm. A commonly used initial sensitivity is 7 μV/mm (Sinha et al., 2016). When the numerical value of the gain is increased, a higher magnitude of voltage is needed to deflect the curve the same distance, and the EEG trace looks therefore smaller. For example, a 100-μV signal deflects the trace on the screen by 10 mm, if the sensitivity is 10 μV/mm. The same signal only leads to a deflection of 5 mm if the sensitivity is 20 μV/mm. Conversely, when one decreases the value of the gain, one “magnifies” the signal on the screen, thus a 100-μV signal, with a sensitivity of 5 μV/mm, leads to a deflection of 20 mm on the screen. Using a cursor, one can measure the precise value of change in voltage and amplitude of a signal.
Thus, digital EEG offers the possibility to zoom-in or zoom-out, both on the horizontal and the vertical scales, for optimal visualization of EEG patterns.
Montages
The arrangement of EEG channels on the display, defined by the active and reference electrodes, are called montages (Kane et al., 2017). The voltage recorded by the EEG in each channel is the difference in electric potentials between the active and the reference electrodes. Digital EEG machines record voltage using a common recording reference electrode (CRR) usually placed somewhere between Cz and Pz (Sinha et al., 2016). For example, in the channel where C4 is the active electrode, the voltage measured is C4-CRR; for P4 this is P4-CRR and so on.
After analogue-digital conversion, these values are re-montaged. One of the advantages of digital EEG is that the same time epoch can be displayed offline in different montages. Even for the digitally re-montaged EEG, there is need for a reference in each EEG channel. Depending on the type of reference, there are two types of montages: those that use scalp electrodes as reference (bipolar montages, referential montages), and those that use computed values as reference.
In referential montages, each scalp electrode can be referenced to the same scalp electrode. This reference electrode is usually located at Cz (see figure 7A) or over an earlobe such as A1 or A2 or as A1 connected to A2. In the old analogue EEG machines, montages were achieved by physically connecting these two electrodes (inputs) to the amplifier channel inputs. For digital EEG, these channels can be calculated from the values of the voltage measured at the scalp electrodes, using the CRR. For example:
Upon subtraction, CRR cancels out, and the value, C4-Cz, is the same as the one that would have been recorded had these channels been physically connected. In referential montages, the voltage depends equally on the active and reference electrode. Ideally, the reference electrode is “inactive”, so that the channel only reflects the potential at the active electrode. However, there is no place on the scalp that is electrically inactive or neutral. Cz records large potentials during sleep, which then show up in all channels, making the EEG difficult to read. A1 and A2 are close to the basal part of the temporal lobe, and record potentials from these regions, which therefore “contaminate” all channels when these electrodes are used as reference. Placing an electrode outside the head is an option, but that results in large ECG artifacts in all channels.
In bipolar montages, electrodes are grouped two-by-two in tandem in a sagittal or longitudinal direction (anterior-to-posterior) or in a coronal or transverse direction (left-to-right) (see figures 7B, C). In the longitudinal bipolar montage, which is often referred to as “double banana” montage, one starts from an anterior electrode (Fp1 / Fp2) as the active electrode and connects it to the next posteriorly located electrode (F3 / F4) as the reference electrode. In the next channel, the electrode which was the reference in the previous channel (F3 / F4) becomes the active one, and the next posteriorly located electrode (C3 / C4) becomes the reference, etc. (figure 7B). In the transverse montage, channel one starts from the left, and proceeds with the electrode pairs to the right (figure 7C). In digital EEG, the electrode pairs are not physically connected, but recalculated. For example:
Common average montage uses, as reference, a computed or calculated value, for example, the average of all electrodes. The voltage of each electrode is divided by the number of electrodes on the head, and then a sum of these is calculated (Scherg et al., 2002).
CA is the common average reference value, n is the number of electrodes on the scalp.
For example, for F4 in a common average montage, the voltage is calculated as follows:
Again, the value of the CRR cancels out.
To avoid large-voltage eye blink artifacts, Fp1 and Fp2 can be excluded from the average calculation, however, that subsequently distorts the scalp topography of the voltage maps. In contrast to the referential montages where a scalp electrode is used as a reference, signals shown by common average do not depend on a single active electrode. For example, in the 25 electrodes of the standard IFCN array, the contribution of any one particular electrode to the common average value is only 4% (100% divided by 25). As the reference value gives half of the signal in a channel, the contribution of one scalp electrode which goes into the common average is only 2%. The contribution of the active electrode, from which the common average is subtracted, is 50% of the signal.
The head is almost a closed spherical volume in which the currents flow. Hence, the negative potentials on the scalp have approximately the same magnitude as the positive potentials. The sum of all potentials on the surface is close to zero. By taking the average of all recording electrodes, one attempts to achieve a reasonable baseline. Relative to this baseline, some electrodes are negative, others are positive, and their sum is close to zero potential. However, the EEG electrodes are not evenly distributed on the head. In the 10-20 electrode placement scheme, there are more electrode samplings from the upper part (top) of the head, which gives an upward-shift of the zero-voltage line. This bias can be reduced if the electrode array includes the inferior-temporal electrodes (Seeck et al., 2017).
Physicians not familiar with the biophysical background of the montages, and without training in signal analysis, often put common average into the same category as the referential montage, because all active electrodes share the same reference. However, the way the reference is calculated in common average is completely different than in referential montage, and these montages should not be considered equivalent. There are many misconceptions about common average montage. The main one is that they show “ghost potentials” generated by the way the reference is calculated. These misconceptions are spurred by the fact that the common average more clearly shows positive potentials than bipolar montages. However, this is not a mathematical “ghost” but rather a biophysical necessity. There must be as much positivity on the scalp as there is negativity.
Another way of calculating the reference value is used in source derivation or Laplacian montage. The aim of this montage is to suppress the effect of volume conduction and show the signals mainly at those electrodes where their amplitude is the largest. In this montage, a reference value is calculated separately for each active electrode on the scalp. Thus, source derivation is different from common average in the sense that the reference differs from channel to channel. The reference is calculated from all the scalp electrodes surrounding the active electrode. However, the contribution of the surrounding electrodes to the reference is not equal since those electrodes that are closer to the active electrode are weighted more than those further from the active electrode. For example, the reference electrode for P4 is calculated by this weighting: 0.14xCz + 0.18xPz + 0.18xO2 + 0.18xP8 + 0.14xT8 + 0.18x- C4. The distance that provides the weighting is calculated on a sphere (Laplacian). Electrical activity is subtracted by the source derivation surrounding the active electrode. One can think of this montage as a more complex three-dimensional version of the bipolar montages. This montage enhances the activity that is unique to the active electrode of the channel.
There are also several drawbacks to this montage. Widespread potentials, such as regional slowing, are much attenuated in source derivation because of the way the reference potential is calculated. Active electrodes that are at the “edge” of the array can have erroneous reference values, as they are not completely surrounded by other electrodes, hence the reference is imprecise.
A virtually “reference-free” montage can be computed by calculation of the voltage over the surface of the head at predefined equidistant positions covering both the upper and lower part of the head, like a sphere. Setting this integral to zero gives the reference-free montage. In most cases, the signal in this montage resembles the one in common average (Scherg et al., 2002).
Using a more advanced computation and spatial filtration of the signals, one can estimate the current flow in pre-defined regions of interest and display the EEG as the change in time of the current flow in that specific region. Thus, one can display the EEG in the source space rather than in the sensor space, as with the classic montages. Using 25 sub-lobar regions of interest, one can virtually represent the electric activity in the brain (Scherg et al., 2019).
Estimation of the source using EEG montages
In this section, we explain how the location and orientation of the cortical generator or source determines the waveforms in different EEG montages, and how that can be used to estimate the location of the source.
As we described earlier, a radially oriented source causes a large amplitude and relatively circumscribed negativity surrounded be low-amplitude and widespread positivity (figure 2). In a bipolar montage, the electrode overlying the highest negative potential, i.e., peak-negativity, will be more negative than the electrodes preceding or following it in the bipolar chain of electrodes. In the channel, where the electrode with peak negativity is the reference electrode, the signal is positive or downward because the active electrode is more positive than the reference (figure 8A). In the next channel, where the electrode with peak negativity is the active one, the signal is negative or upward-going. This pattern of signals “crossing” each other is called a “negative phase-reversal” (figure 8A). When the scalp area with peak negativity is more widespread, such that two electrodes are overlying the peak-negativity, the channel connecting them shows an isoelectric or flat line and the phase reversal is between the channels preceding and following the channel with isoelectric line (supplementary figure 1). Obviously, when the peak negativity is at the end of an electrode chain, there will not be a phase reversal but rather just a negative or positive deflection in the first or last channel in the chain. Bipolar montages are quite good for spotting the peak negativity. However, the widespread, low-amplitude positivity is typically not visible in bipolar montages, because the difference in potential between the neighboring electrodes is so small (figure 8A) and most experts reading EEG only in this one montage are not even aware of the existence and/or relevance of these positive potentials. In common average montage, the picture is more straightforward; large-amplitude negative (upwards) deflections occur at the electrode overlying the peak negativity, and low-amplitude positive (downwards) deflection at the electrodes overlying the widespread low-amplitude positivity (figure 8B).
A tangentially oriented source, for example one located in the wall of a sulcus, causes two well-defined poles, a negative and a positive one on the scalp (figure 2). In bipolar montages, these peaks will often be at the opposite ends of the electrode chains, and the gradual transition between the peak negativity and peak positivity will be observed, but not the typical topography consisting of negative and positive poles on the scalp (figure 9A). In common average montage, electrodes overlying the negative peak have large negative deflections, and electrodes overlying the peak positivity have large positive deflections (figure 9B).
It is important to emphasize that all montages have their advantages and disadvantages (table 1 table 1). A skilled reader quickly and easily switches between several montages when spotting an abnormality. Gibbs and Gibbs (1964) considered the following disadvantages for the bipolar montages; their tendency to
- –reduce voltage sometimes to the point of vanishing, creating complex waveforms;
- –make it difficult to recognize a “true” electrical sign;
- –and create out-of-phase relationships that are hard to distinguish from true physiological out-of-phase voltage (Gibbs and Gibbs, 1964).
The authors also pointed out that many patterns are well-delineated and were first seen on referential montages. These include sleep patterns, 14 and 6-Hz positive spikes and many of the specific seizure patterns. One can often encounter the opinion that common average has the “tendency to erroneously show generalized discharges” when several electrodes are active in one region of the scalp, such as a focal ictal rhythm, even when the signal is strictly unilateral. This is not true. The signals of the two sides are out of phase, and one needs to inspect the scalp distributions of both polarities to correctly estimate the source.
When two adjacent electrodes, by mistake, become shorted or connected by a “salt bridge”, i.e. via the conducting paste, they record the same potential. The bipolar montage shows an isoelectric (flat) line in the channel connecting these electrodes, which is immediately noticed (and hopefully corrected) by the technician. However, if the shorted electrodes are displayed in common average montage, the potentials at both electrodes will be different from the value of the common average reference, generating a signal, and the salt-bridge may be overlooked. It is recommended that, at the beginning of the recording, the technician inspects the EEG in longitudinal and transversal bipolar montages, to detect for possible technical failures (Sinha et al., 2016).
Estimation of the source using voltage maps
When the cortical source has a radial orientation, localization is straightforward since the peak negativity or the site of a phase reversal in bipolar montage, or the largest negative deflection in common average montage, is overlying the source. However, when the source has radial orientation as in a source located in the wall of a sulcus, the peak negativity on the scalp is distant from the source (figures 2 and 9). Without taking into account the scalp distribution of positivity, one does not know the orientation of the source. The best way for visual estimation of the cortical source is based on inspection of the voltage topographies, as shown by voltage maps. Most digital EEG systems have this tool.
Voltage maps (figures 10 and 11) show, in two or three-dimensional drawings, the distribution of the negative and positive potentials over the scalp at any distinct point in time, marked by the cursor (Scherg et al., 2019). This is referred to as “voltage or scalp topography”. Color codes depict the potentials where blue is for negative and red is for positive potentials. Deeper nuances depict larger amplitudes of the potentials. The voltage values on the scalp in the areas between the electrodes and outside the electrodes are estimated using interpolation and extrapolation, respectively. Thin lines in the maps indicate the gradual transition between the two poles where the amplitude difference (in μV) between the negative and the positive peak is divided into equal steps. The area between two adjacent lines has voltage values within one such step.
The topography corresponding to the radial orientation shows relatively circumscribed, large-amplitude negativity, surrounded by low-amplitude, diffuse positivity (figures 10A and 11C). In this case, the source is under peak negativity. When the topography shows two well-defined poles, a negative one and a positive one (figures 10B and 11A, B), the orientation is tangential. In this case, the source can be localized using the following steps:
- –connect peak negativity with peak positivity;
- –and find the area with highest gradient (where the isoelectric lines are closest to each other).
The source is under this area. Additional information can be inferred from the orientation of the polarities, since the surface of the cortical generator is towards the negativity.
Provocative methods that increase the diagnostic yield
Provocative maneuvers are part of the standard EEG recording procedures. Hyperventilation lasting 3-5 minutes (Craciun et al., 2015) should be used except when contraindicated by recent intracranial hemorrhage, significant cardiopulmonary disease, sickle cell disease or trait, or inability or unwillingness of the patient to cooperate (Sinha et al., 2016). Included in the category of contraindication are symptomatic cerebral vascular disorders, including significant obstructive vascular complications either related to underlying cerebral vascular disease or inflammatory vasculitis such as acute symptomatic or chronic Moya-Moya disease. The EEG recording should be continued for at least two minutes after cessation of over-breathing, as abnormalities can occur in the post-hyperventilation period. Significant generalized slowing is frequently seen in young patients which resolves during the first few minutes after hyperventilation is concluded, and is considered a normal variant. Hyperventilation activates epileptiform discharges, most importantly the bilateral, synchronous 3c/s spike-and-slow waves in children with absence epilepsy. Regional slowing over a structural focal abnormality may also be accentuated during hyperventilation.
Photic stimulation should also be used to assess photosensitivity by triggering a photoparoxysmal response. As the effect of hyperventilation might persist during the period after cessation of over-breathing, photic stimulation should be performed before hyperventilation, to separate the effect of the two maneuvers. Photic stimulation should be performed in a room with dimmed lighting using a lamp placed 30 cm from the patient's face (Sinha et al., 2016). Patients should be checked for eye-closure sensitivity which is defined as precipitation of epileptiform discharges due to closure of the eyes. In patients with occipital lobe epilepsy, it is recommended to assess “fixation-off sensitivity”, i.e. the appearance of epileptiform discharges triggered by suppression of central vision and fixation, which can be obtained by eye closure, complete darkness, or Frenzel lenses.
The diagnostic yield of the EEG can be increased by including sleep in the recording, as some EEG abnormalities can be evoked during sleep (Meritam et al., 2018). In small children, sleep is usually achieved in the postprandial period or induced by medication given before the recording (melatonin). Sleep deprivation prior to the sleep recording also has a facilitating / precipitating effect. In patients with suspected juvenile myoclonic epilepsy and West syndrome, the recording should also include a period after awakening.
Specific provocative maneuvers might be necessary in some patients, and including specific facilitating/precipitating events may be very useful. For example, in patients with reading epilepsy, the whole recording can be normal, unless the specific provocation of reading is included. Specific tests are useful not only in patients with reflex epilepsy, but also in patients with idiopathic (genetic) generalized epilepsy, who often show reflex epileptic treats (Koepp et al., 2016).
Types of EEG recordings
A standard (“routine”) EEG recording should include at least 20 minutes (Sinha et al., 2016) of artifact-free period, as well as adequate provocative maneuvers, as described above. Shorter recordings significantly decrease the chance of recording abnormal EEG patterns that occur transiently (Craciun et al., 2014). The recordings should include periods when the eyes are open and when they are closed. When the patient cannot cooperate, eye closure/opening can be performed manually by the technician using the surrounding soft tissue. In the pediatric population, the use of a cuddly toy can be helpful.
Sleep EEG recordings are usually performed in patients who previously had a normal or non-informative standard EEG recording. Often, it is the first EEG in patients with poor cooperation during a standard awake recording (infants). Sleep recording is recommended systematically up to the age of five years (Kaminska et al., 2015), as sleep minimizes the presence of artifacts due to a lack of cooperation, and supplies supplementary information on maturation of brain electrical activity. To obtain sleep in pediatric patients, it is important to organize the EEG appointment at the time of the patient's usual daytime nap and tell the parents to avoid sleep on the way to the EEG department. Oral melatonin can also be very useful and should be given at the beginning of the recording after the set up (Eisermann et al., 2010). At least 30 minutes of sleep is needed to achieve optimal diagnostic yield (Craciun et al., 2014). In some patients, the period after awakening has significant diagnostic relevance; for example, in patients with juvenile myoclonic epilepsy and in patients with West syndrome. Therefore, the recordings should be continued after the patient has woken up, for at least 15-20 minutes, when these conditions are suspected. Sleep can be achieved spontaneously, for example, by scheduling it to a post-prandial period (infants). Alternatively, sleep can be induced by partial sleep deprivation which itself is a provocative maneuver or by medication such as melatonin.
Short-term video-EEGs (1-8 hours) are indicated for recording seizures in patients with high seizure frequency, usually with a rate of several seizures per day. Most modern digital EEG systems have integrated video cameras and therefore this recording is technically simple.
Long-term video-EEG monitoring is performed either in hospital (epilepsy monitoring units) or as an ambulatory EEG (home video-EEG telemetry). The recordings are usually longer than 24 hours and can last several days and up to weeks on rare occasions. The goal of these recordings is to document the electro-clinical phenomena (EEG and semiology) during the patients’ habitual clinical events. This is the gold standard for diagnosing and characterizing seizures and paroxysmal clinical events, and is indicated for diagnosis, classification, monitoring of seizure frequency and presurgical evaluation. Continuous EEG (or video-EEG) recordings are performed: in critically ill patients, in the intensive care unit, for diagnosing non-convulsive status epilepticus, for prognosis and monitoring increased risk of seizures and for delayed cerebral ischemia in patients with subarachnoid hemorrhage.
In selected patients undergoing presurgical evaluation, when the epileptic focus to be resected cannot be localized with sufficient confidence using non-invasive methods, invasive EEG monitoring with intracranial electrodes is indicated using either subdural electrodes (strips and grids) or depth electrodes (stereo-EEG).
Conclusion and key points
EEG is the most often used functional investigation method in patients with seizures and suspected epilepsy. It is important that physicians reading EEG understand the basic biophysical phenomena behind EEG signal generation and the technological aspects of EEG recording.
- –EEG signals are generated by the summation of postsynaptic potentials along the apical dendrites of neurons that reside in layers IV-V.
- –Volume conducted currents to the surface of the scalp result in a topography of positive and negative potentials, depending on the position and orientation of the cortical generator.
- –The standard EEG electrode array of the International Federation of Clinical Neurophysiology consists of 25 scalp electrodes.
- –Digital EEG is recorded with a common reference electrode. The analogue electric signals are filtered, converted to digital signals and re-montaged.
- –EEG is displayed as an oscilloscope, in which changes in voltage are plotted on the vertical axis with negative polarity upwards against time on the horizontal axis.
- –Various montages are available for displaying signals at the active electrodes, using either another scalp electrode (bipolar and referential) or a calculated value (common average, source) as reference.
- –One should take advantage of the benefits of digital technology when reading EEG; these include the ability to change montages, filters, time-resolution and gain, and to optimize visualization of the EEG patterns.
- –For visual estimation of the cortical source, the distribution of negative and positive potentials is inspected. This is most effective using topographic (voltage) maps.
- –Provocative maneuvers such as hyperventilation, intermittent photic stimulation and sleep can increase the diagnostic yield of EEG recordings.
- –Various types of clinical EEG recordings are available: standard, sleep, short and long-term video-EEG recordings, and invasive (intracranial) recordings.
Case box 1
A seven-month-old girl with normal birth and development started having occasional episodes described by the parents as small jerks. They were reassured that this was just an intestinal cramp (baby colic). However, the jerks, which included brief stiffening of the arms and legs, increased in frequency and several jerks occurred in clusters. The baby became irritable and changes in the sleep-wake cycle were noted by the parents, sleeping more during the day and less during the night. An EEG was requested. However, the recording was hardly interpretable due to almost continuous movement and muscle artifacts. The baby did not fall asleep during the recording. The few, brief epochs not obscured by artifacts were normal. A new recording was requested, after administration of melatonin. The sleep recording showed high-amplitude (>300-μV) synchronous, disorganized delta activity, with intermixed multifocal spikes, indicating hypsarrhythmia. West syndrome was diagnosed and treatment with vigabatrin was started.
Comment: In patients with West syndrome, the diagnosis is often overlooked at the beginning of the symptoms, when the developmental delay is not yet obvious and the spasms are infrequent and not in clusters. The standard awake EEGs are often severely disturbed by artifact and can be normal / unrevealing. Sleep recordings increase the chance of identifying hypsarrhythmia.
Case box 2
A 19-year-old male college student started experiencing jerks in the facial muscles, when studying for exams. The jerks stopped when he took breaks from studying. However, on one occasion, he continued studying and a generalized convulsion was witnessed by his roommate. The patient was admitted to the hospital and a standard EEG was performed. The 30-minute recording, which included a short period of drowsiness and Stage N2 sleep, was normal, and the patient was dismissed from the hospital. However, the jerks continued to occur while studying. A second EEG was requested, including specific provocative maneuvers. The patient brought his textbook with him and he read during the EEG recordings. After three minutes of reading, perioral muscle-jerks appeared, with EEG correlate (polyspike-and-wave). Reading epilepsy has been diagnosed.
Comment: In patients with reflex epilepsy, EEG is often normal, unless the patient is exposed during the recording to the specific provocative maneuver that triggers the seizures.
Supplementary data.
Summary didactic slides, supplementary figure and video sequence are available on the www.epilepticdisorders.com website.
Disclosures.
None of the authors have any conflict of interest to declare.
![]() This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License
This work is licensed under a
Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License