Epileptic Disorders
MENUAwake craniotomy for epilepsy surgery on eloquent speech areas: a single-centre experience Volume 23, issue 2, April 2021
Surgical management of medically refractory epilepsy can be tricky when the epileptogenic zone (EZ) is located very near to, or even within eloquent areas of the brain regarding the balance between the extent of epileptic focus resection and potential postoperative neurological deficits. Yet, complete resection of the seizure focus close to eloquent cortex, while carrying the highest risks for new neurological deficits, offers the best postoperative seizure control [1-3].
Accurate seizure focus localization is essential for favourable surgical outcome in epilepsy surgery. Many seizure focus localization techniques are available including non-invasive techniques, such as scalp EEG (electroencephalography), MRI (magnetic resonance imaging), FDG-PET (fluorodeoxyglucose positron emission tomography), MEG (magnetoencephalography), fMRI (functional MRI), as well as, when necessary, invasive intracranial recordings using SEEG (stereo-electroencephalography) or subdural grids. For the latter, preoperative cortical mapping is essential to accurately identify functional areas in eloquent cortex in order to guide the extent of resection [4].
Regarding the language areas, essential language function is located in the pars opercularis of the inferior frontal gyrus (Broca's area) and in the posterior superior temporal gyrus (Wernicke's area), but stimulation mapping, however, has demonstrated that essential cortical language sites can be variably located in the frontal, parietal, temporal, and insular lobes [5, 6]. This is why it is crucial to establish as precisely as possible the location of speech areas in each individual patient. In many cases, these data can be clearly obtained from the pre-operative investigation data, largely from the combination of clinical, fMRI, and preoperative cortical mapping data (if invasive recordings have been performed). In some cases, however, despite the use of all possible preoperative functional investigations along with pre-surgical cortical mapping by depth (or subdural) electrodes, the decision to propose resective surgery to the patient, without taking unacceptable risks of post-operative language impairment, may be challenging or even impossible, either because the extent of nearby speech areas may not be accurately delimited, or because although there may be clear delineation, there may also be partial overlap between the speech areas with the epileptic zone (EZ) to be removed.
For such patients, rather than excluding them from surgery or risking incomplete focus resection, the question arises as to whether resective epilepsy surgery under awake conditions should be performed, similar to that previously proposed for glioma surgery. Using an awake craniotomy technique for language cortex localization during resective epilepsy surgery is something that has not yet been fully reported in the literature [7].
We present a retrospective series of 17 patients presenting with intractable epilepsy, who had undergone awake craniotomy with cortical language mapping in order to perform the most complete focus resection under local anaesthesia. We aimed to investigate this technique based on resections of (non-glioma) epileptic zones located in different language areas, and further determine its efficacy regarding seizure control and functional outcome.
Patients and methods
Patients
The authors performed a retrospective study of a cohort composed of all patients with intractable partial seizures who were operated on for resective epilepsy surgery in the Department of Functional Neurosurgery at Pierre Wertheimer Hospital for Neurology and Neurosurgery of Lyon, France, in the last 10 years (from 2010 to 2019). During this period, 344 patients benefited from a resective epilepsy surgery procedure. Among them, 191 presented with a mesial temporal lobe epilepsy syndrome and underwent a standardized anterior temporal lobectomy, which does not require any per-operative cortical mapping. Among the remaining 153 non-temporo-mesial cases, 64 underwent a tailored corticectomy in different areas of the dominant hemisphere, and 17 of them finally met our inclusion criteria and were operated on under awake conditions for language function monitoring.
Patients were included in the study if preoperative investigations (seizure semiology, fMRI, and neuropsychological assessment, as well as SEEG stimulation mapping in those who underwent SEEG) revealed the EZ to be sufficiently closely related to eloquent speech areas to make us decide to perform surgery under wakefulness. In cases of doubtful pre-operative localization of language area, we considered a priori that any EZ located in, or next to, the posterior two thirds of the dominant F3 gyrus, the dominant supra-marginalis gyrus, or the dominant insula, was potentially involved in language function.
Patients excluded from being operated on using this technique were children less than 14 years of age, morbid obese patients, patients with severe dysphasia, patients with psychiatric history, emotional instability or severe anxiety disorder, and patients with impaired cognition or intellectual disability.
Clinical information was retrospectively obtained from the patient medical records, including imaging data. All patients were informed about their enrolment in our study, and the study was approved by the institutional ethical committee of scientific research.
Methods
All patients underwent primary investigations to localize and lateralize the EZ. Interictal EEG, video scalp-EEG, MRI, and PET-FDG, were performed in all patients. Invasive EEG recordings (SEEG) were needed in 13 patients (76.4%), as the data provided by the non-invasive part of the pre-surgical investigations were not sufficiently congruent to propose resective surgery. By definition, in this study, the data provided by the (non-invasive and/or invasive) presurgical workup in all of the 17 patients reported here led to a clear delineation of the epileptogenic focus, but were not sufficient to safely decide to operate, either because the exact extent of the speech areas could not be delimited with sufficient accuracy, or because there was a certain overlap of the speech areas with the epileptogenic focus. Preoperative fMRI was performed in all patients using a blocked design (alternating task and control periods) over six functional scans. Three processing tasks were used: semantic fluency, verb generation, and sentence comprehension, and the resulting statistical maps were projected onto the patient's T2*-weighted images. Neuropsychological evaluation and preoperative basic language and sensorimotor assessment were also performed in all patients. The neuropsychological evaluation played a major role, along with careful interview by the surgeon and a trained anaesthesiologist, in assessment of feasibility of the awake procedure, and of its acceptance by the patient.
In patients who underwent SEEG, direct electrical stimulation using depth electrodes was used for mapping the eloquent brain areas and attempting to delineate the relationship between the epileptic zone to be resected and the language network. As already published by our group [8], SEEG stimulations were performed over two or three sessions of a half to one hour by the neurologist who knew the patient case. Stimulations were performed between two contiguous contacts in bipolar and biphasic manner. There were two types of stimulation: low-frequency stimulation (shock stimulation; parameters: frequency of 1 Hz, intensity of 0.5 to 4 milliamps, shock duration of 0.5 to 3 msec, stimulation duration of 20 to 60 seconds), and high-frequency (train) stimulation (parameters: frequency of 50 Hz, intensity of 0.5 to 5 milliamps, shock duration of 0.5 to 3 msecs, and duration of 3 to 8 secs).
No new antiepileptic drugs were added prior to surgery, and patients were under their routine antiepileptic drugs previously adjusted by the neurologists, according to each case, despite being intractable to these drugs.
Prior to the surgery, the patients were informed about the surgical intervention plan, the possible risks and complications of surgery, as well as possible discomfort associated with craniotomy. Possible inconveniences (forced position on the operating table, the probability of occurrence of aphasia, uncontrolled muscle contractions or movement blockage during cortical stimulation, or seizure development) were described to the patients. Informed written consent was obtained from each patient.
Once in the operating room, patient monitors were applied (blood pressure cuff, arterial line), and all patients were given supplementary oxygen by nasal cannula and scalp block was performed with levobupivacaine, followed by local anaesthesia infiltration along the incision line. Monitored sedation with dexmedetomidine or propofol plus remifentanil was used and adjusted according to each situation. In our last two cases, the technique of hypnosis aided awake resection, which aims to optimize the comfort and well-being of the patient, reducing the pain and anxiety during the surgery [9]. The anaesthesia procedure was performed by a specialized neuro-anaesthesia team, with good communication between the surgeon and neurologists throughout the mapping and monitoring procedure.
After positioning, the patient was registered in the neuronavigation system (Medtronic ® Stealth Station, Minneapolis, MN, USA), to plan for incision and craniotomy. As this was epilepsy, rather than glioma surgery, the bone flaps were minimized in size, and centred with neuronavigation on the EZ specifically, and not on the whole suspected language area.
After incision, opening of the dura, and exposure of the brain surface, functional cortical mapping was performed using Nimbus ® (Innopsys ltd, Carbonne, France) intraoperative neurophysiological monitoring system and bipolar probe with 1-cm spacing between the tips. We used a pulse width of 1,000 μsec, frequency of 50-60 Hz, and train duration of 2-4 secs. We started with the lowest intensity of 3 mA, and increased the intensity gradually by 3 mA, guided by electrocorticography (ECoG), as long as no after discharges were detected by the ECoG. In order to minimize any risk of developing per-operative seizures, no more than three successive stimulations were performed to the same point, and an attempt was made to strictly minimize the number of stimulated sites. The maximum current intensity used was 9 mA, even in absence of after-discharges, after which the mapping at this point was considered negative. In cases of positive cortical mapping (the presence of a trained neurologist in the operating room allows to accurately assess the exact nature of any speech disturbance), resection was started either from the nearest neighbouring sulcus to the positive point, or a safety margin of 1 cm was left around the positive point if the neighbouring sulcus was further than 1 cm. The area to be resected was thus delineated according to positive and negative cortical stimulated sites. Then, continuous monitoring of the language functions (speech and reading) was performed throughout the procedure with continuous discussion and testing for language functions while performing the cortical resection itself, being watchful to any sign of speech impairment or reading difficulty, which would imply an immediate interruption of tissue removal in the area. This approach to maximize cortical resection while maintaining discussion with the patient makes any subcortical electrical stimulation, to our mind, unnecessary.
Postoperatively, patients were admitted to the ICU for overnight close monitoring, and all patients underwent brain CT on the second day of surgery and MRI was planned later on, during the follow-up visits, according to each patient case.
On the second day after surgery, all patients were transferred to the ward, and were asked about their emotional experience during the awake surgery. None of the patients reported any bad emotional experience regarding the awake resection, and declared being ready for second awake surgery in the future, if needed.
Baseline patient clinical characteristics (gender and age), anatomical location of the epileptic zone, and MRI data were compared between the patients. The relation between preoperative cortical stimulation, intraoperative cortical stimulation, seizure outcome and postoperative functional state was analysed. Possible correlations between the intraoperative cortical stimulation data and seizure outcome was statistically assessed using the Chi square test.
Results
Between November 2010 and April 2019, a total of 17 patients (10 males, seven females) underwent resective epilepsy surgery under awake conditions in our institute. All patients presented with intractable left focal seizures; the mean age at seizure onset was 10.3 years (from two to 22 years) and the mean age at the time of surgery was 26.4 ± 12 (standard deviation [SD]) (range: 16 to 40 years).
The EZ was frontal in seven patients (41.2%), insular in five patients (29.4%), and latero-temporal in five patients (29.4%). Different epilepsy aetiologies were encountered radiologically, including non-lesional epilepsy in three patients (17.6%), tuberous sclerosis in one patient (5.8%), hypoxic ischaemic insult in one patient (5.8%), DNET in two patients (11.7%), FCD type 2 in four patients (23.5%), and other malformations of cortical development (MCD) (cortical thickening, dysplasia, etc.) in six patients (35.2%).
Functional MRI revealed language representation to the left side in 12 patients (70.5%), bilateral representation, but predominantly on the left side, in three patients (17.6%), and bilateral representation, but predominantly on the right side, in two patients (11.8%).
Preoperative direct electrical stimulation mapping of the language areas by SEEG was performed in the 13 patients (76.5%) in which SEEG was indicated for EZ localization. SEEG stimulation mapping revealed certain language blockage over or on the borders of the area to be resected in four cases (Patients 4, 11, 12, 17) and doubtful language blocking (which could not be confirmed due to symptoms of stimulation-induced seizures) in one case (Patient 10).
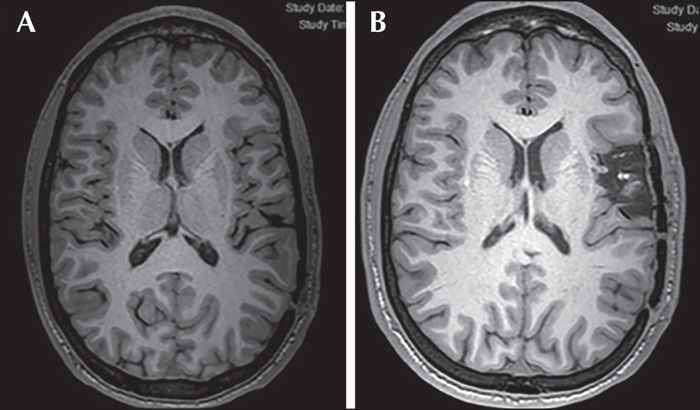
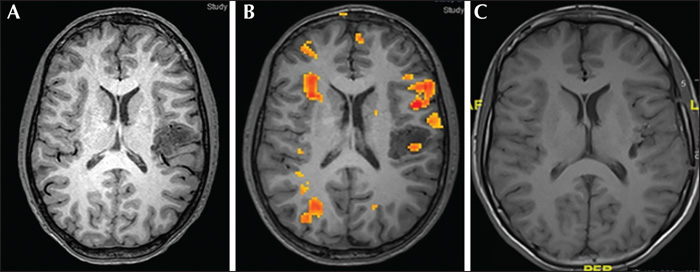
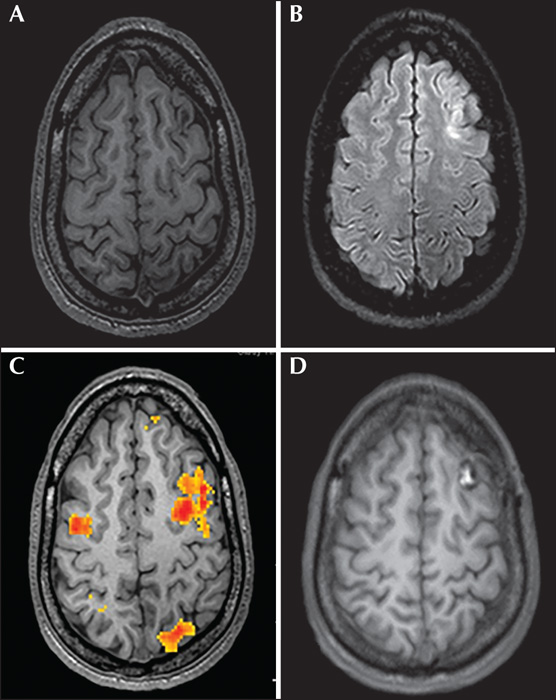
Intraoperative cortical stimulation mapping of the suspected speech areas under awake conditions revealed speech abnormalities in seven patients (41.2%), ranging from mild transient verbal changes and dysphasia in four cases (Patients 3, 7, 8, and 16) (57%), to complete aphasia in three cases (Patients 10, 12, and 13) (43%). Table 1 shows the EZ characteristics and fMRI data and demonstrates the differences between the results of preoperative stimulation mapping and those of intraoperative stimulation mapping. Some illustrative examples are shown in figures 1-3.
Speech arrest revealed by direct cortical stimulation made the neurosurgeon and the neurologists change their decision intraoperatively regarding the extent of resection in two patients (11.7%). In one patient (Patient 10), the surgery was aborted because stimulation revealed complete aphasia over the area to be resected (posterior inferior frontal gyrus) and this patient had doubtful speech arrest during SEEG stimulation. In the other patient (Patient 12), the cortical stimulation induced complete aphasia over the posterior part of the area to be resected, located in the posterior superior temporal gyrus. In this latter case, an incomplete anterior resection was performed. In the last case (Patient 13), in which intraoperative stimulation resulted in complete aphasia, the decision was taken, despite this, to perform a very strict lesionectomy through a new trajectory, different from that already planned, while discussing with the patient. This could finally be performed without any intraoperative or postoperative speech disturbance, and complete resection was achieved. The mild or inconstant verbal changes observed after intraoperative stimulation (Patients 3, 7, 8, and 16) did not lead to any change in the surgical plan; resection was performed as planned, while maintaining discussion with the patient. Thus, by comparing the previously planned resection limits with the actual resection performed, complete resection was achieved in 15 patients (88%), incomplete resection in one patient (5.8%), and no resection in one patient (5.8%).
A stimulation-induced intraoperative seizure was recorded in one patient (5.8%). The seizure was very mild in the form of brief hemifacial clonic seizures which aborted spontaneously within a few seconds without any consequences. No intra-operative complications (vascular injury or injury to important structures) were recorded in our series. The mean duration during which the patient had to be fully awake, alert and actively participate with the neurologists (from opening the dura to the end of resection) was 75 mins (range: 50 to 110 mins).
Apart from the surgery that was aborted, as mentioned before, among the 16 patients who had completed the procedure to the end, new-onset neurological deficits during resection, while the patient was still awake, were encountered in two patients (12.5%) (Patient 7, in the form of expressive dysphasia and distal motor weakness) and (Patient 13, in the form of mild facio brachial weakness and mild dysarthria). Postoperative new-onset neurological deficits were encountered in another two patients (12.5%) (Patient 1, in the form of expressive aphasia) and (Patient 5, in the form of mild reading difficulty). Postoperative CT revealed mild brain oedema at the site of surgery and brain dehydrating measures were considered.
Thus, in total, we encountered new-onset neurological deficits in four patients (25%), however, all these deficits were regressive and transient, and neurological examination did not reveal any neurological deficits in all these patients after six months. A neuropsychological assessment was performed in all cases between 6 and 12 months postoperatively, which did not reveal any deterioration when compared to the preoperative period. No patient, when interviewed at that time by the surgeon or the epileptologist, reported a bad emotional experience regarding the previous surgical procedure.
The mean follow-up period was 5.7 years. At the last follow-up visit for each patient, the seizure status was assessed according to Engel's classification. Seven patients were classified as Engel Class Ia (43.7%), four patients Engel Class IIa and IIb (25%), and five patients Engel Class IIIa (31.2%); the patient whose surgery was aborted, as already mentioned due to the fact that it was impossible to perform the resection, was excluded from our postoperative assessment results. Therefore, in summary, 68.75% (11/16) of the patients (Class 1 and 2) had undergone noteworthy improvement or were cured after surgery.
The five patients with poor seizure control after surgery (Engel Class IIIa) and the patient for whom the surgery was aborted were further rediscussed regarding the possibility of planning for reinvestigations and second surgery if possible.
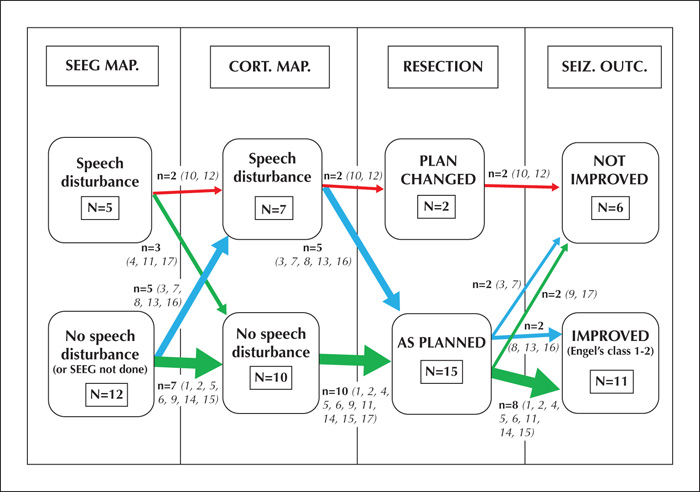
Assessment of the relationship between cortical stimulation mapping data (either negative or positive mapping) and seizure outcome revealed that mapping was negative in 10 patients, among whom eight patients had good seizure outcome postoperatively (80%), and positive in seven patients, among whom three patients had good seizure outcome (42.8%). This means that negative mapping is more likely to be associated with good seizure outcome. However, this difference was not statistically significant (p = 0.2), based on the chi square test. These finding are summarized in figure 4.
Discussion
Our study shows that the use of an awake craniotomy procedure can certainly constitute added-value for some complex cases of epilepsy surgery, as it allows some otherwise non- or barely non-operable patients to become eligible for resective surgery, with minimal risks of functional complications and a good chance of post-operative improvement regarding seizure status.
Awake craniotomy was first introduced in the field of neurosurgery by Victor Horsley in 1886. It was initially mainly associated with removal of epileptogenic foci and, quite irrespective of the location of the epileptic focus, the use of local anaesthesia became the standard at the majority of surgical institutions worldwide, such that the use of local anaesthesia became synonymous with epilepsy surgery over the early and middle parts of the twentieth century [10-12]. The primary reason for this was probably the dependence on the intraoperative ECoG for the localization of epileptic foci, and the concern of the effect of general anaesthesia on the ECoG in the early part of the twentieth century.
With the advent of modern neuroanaesthesia, modern neuroimaging, and with more reliable presurgical investigation, as well as, later on, neuronavigation, there has been a striking decrease in the number of surgeries performed under local anaesthesia, as these techniques provide sufficient confidence in anatomical and functional localization, making, in most cases, general anaesthesia appropriate and recommended. However, neurosurgery under local anaesthesia continues to be an important technique that should be used in specific situations.
Thus, during the past 20 years, modern developments of awake surgery have been supported mainly by glioma surgeons. Recently, however, because of the development of the field of invasive epilepsy monitoring and of the notion of relationships between epileptic networks and the brain connectome, functional mapping tends to be reconsidered in the field of epilepsy surgery [13-15].
There are some differences between awake craniotomy for glioma and epilepsy. In epilepsy surgery, there is no significant change in the gross cortical anatomy in contrast to glioma surgery, which causes disruption of normal gross cortical and subcortical anatomy. In the case of gliomas, surgery is performed after a long period of tumour-triggered physiological neuroplasticity and at the beginning of epileptogenesis, which allows better recovery for neurological deficits. Conversely, in focal drug-resistant epilepsy, surgery is performed after a long period of epileptogenesis, and in the absence of any lesion-induced neuroplasticity of the neocortex. Moreover, white matter adverse plasticity may have occurred and created new pathological pathways for seizure spreading, different from that found in the normal connectome, rendering the ability to delineate the epileptic zone more difficult, and regression of neurological deficit less likely to occur [7].
Description of language mapping during awake craniotomy for cortical resections of non-lesional or lesional epilepsy (aside from gliomas) in the literature is rare and limited. S. Maesawa et al. [16]presented a study of four cases of epileptic lesions in language areas, operated under awake conditions. However, all cases in this series had image-demarcated lesions (gliosis, gliomas and cavernomas), and ECoG was used in all cases intraoperatively to permit localization of the epileptic zone in the selected cortex. The results showed that for patients with epileptogenic foci in and around the functionally eloquent areas, awake surgery may lead to improved seizure control and limited neurological complications by facilitating maximal lesionectomy while preserving dominant functions. Y.H. Kim et al. [17] presented a study of nine cases with non-lesional neocortical epilepsy in which resection was performed in the Broca's area under awake conditions. Preoperative stimulation mapping using subdural grids was used in all cases in this series. The data demonstrated that awake resective surgery under local anaesthesia with intraoperative functional mapping is an effective and safe treatment option for non-lesional neocortical epilepsy involving the eloquent areas or adjacent areas. However, this study demonstrated that resections in sensori-motor cortex are more tolerable than resections in speech areas. In contrast to these studies, our study included both epileptic lesions other than gliomas (FCD, MCD, DNET, tuber, hypoxic ischaemic cortex) and non-lesional epilepsy. Moreover, preoperative SEEG stimulation language mapping was used in most of our patients.
One might think that operating on patients with intractable epilepsy with awake craniotomy and cortical stimulation mapping carries a much more increased risk of intraoperative seizures which might have unpleasant consequences. However, in our series, mild seizures occurred only in one patient (5.8%). The incidence of stimulation-induced seizures during awake craniotomy for gliomas in the literature ranges from 2.2% to 21.5%. However, as already mentioned, the use of ECoG is essential to detect afterdischarges, i.e. by following the protocol for safe cortical stimulation already mentioned and using ECoG, there is no difference in the risk of intraoperative seizures between surgery for gliomas and epileptic lesions under awake conditions.
Functional MRI is routinely performed in our centre when there is a suspicion of overlap between the EZ and language cortex, which provides preliminary insight into language cortex lateralization and localization. Functional MRI has a number of advantages: it is non-invasive, requires no injection of medication, and carries virtually no risk of complication. However, there remain strong limitations to fMRI scanning in defining atypical and/or essential language areas. Namely, the level of spatial resolution of fMRI data is lower compared to that of typical T1-weighted anatomical MRI, the BOLD signal itself is not yet entirely understood, and the relationship between positive and negative BOLD signals and alterations in neural activity have not been fully elucidated [18]. Essentially, fMRI identifies language regions via behaviour-associated activation, whereas this is achieved via focal disruption for direct cortical stimulation. Consequently, regions activated by functional MRI are more extensive than those elicited by direct cortical stimulation, which could lead to more conservative decisions [19]. This explains why, in our experience, we depend mainly on direct cortical stimulation to plan the boundaries of resection.
Preoperative language stimulation mapping using SEEG was performed in the 13 patients who required preoperative investigation using SEEG, and this revealed certain language blockage around the area to be resected in four patients and doubtful language blockage in one patient. It is, however, noteworthy that in two of the three patients in whom direct intraoperative cortical stimulation over the area to be resected induced complete aphasia, SEEG stimulation mapping revealed certain language blockage on the margins of the epileptic zone to be resected in one patient and doubtful language blockage in the other patient (no SEEG was performed in the third patient). Conversely, in the three remaining patients in whom SEEG stimulation mapping revealed language blockage over or around the epileptic zone, intraoperative direct cortical stimulation did not reveal any kind of language block over or around the area to be resected. Gil Robles et al. [20] compared language mapping using SEEG and intraoperative direct electrical stimulation (albeit in one patient), and the data showed discordance between the modalities. The explanation could be that SEEG stimulation provides a very focal sampling of the brain, which makes it a less sensitive technique than intraoperative cortical stimulation. Therefore, this renders intraoperative cortical stimulation irreplaceable for functional mapping. Thus, we can consider SEEG stimulation mapping as a complementary method to initially assess the connectome prior to surgery which may help focus on the most relevant elements peroperatively[7].
In cases of insular epilepsy, although the insula cannot be considered strictly indispensable for speech function (it has been shown to be involved in speech motor processing and language articulation) [21], we considered the awake state to be necessary in order to minimize any potential risk of postoperative deficit (which could be acceptable in cases of glioma surgery, but not in cases of epilepsy surgery) and to minimize the risk of any injury to white matter tracts involved in language function (especially the inferior fronto-occipital fasciculus) [22]. We gained direct and good access to the insular cortical surface after having removed the overlying operculum. Such a transcortical approach has the advantage of minimizing complications, such as local post-operative oedema, resulting from an involuntary excessive retraction of the opercular cortices, and in any case is necessary because the EZ almost always spreads to the overlying operculum of the involved insular cortex.
This study, however, is not without limitations. Firstly, the time dedicated to intraoperative stimulation was limited. Secondly, direct cortical stimulation led to a high rate of negative language mapping, probably due to the small size of the bone flaps, specifically centred on the EZ and not on the whole suspected language area. Thirdly, the study involved a small number of patients, as well as different types of pathologies causing epilepsy which could affect seizure outcome as well as the extent of resection. However, there are currently few drug-resistant epilepsy cases that require intraoperative direct cortical stimulation of speech areas, even in tertiary epilepsy surgery reference centres, such as ours.
In summary, our results demonstrate that the use of the awake craniotomy technique may enable different types of epileptic zones (lesional or non-lesional), located in or very near to eloquent language cortex and causing intractable seizures, considered in the past to be inoperable, to be resected safely, resulting in noteworthy improvement in seizure outcome (Engel Class Ia or IIa in 68.7% of patients), without causing any permanent neurological deficit.
Supplementary data
Summary didactic slides are available on the www.epilepticdisorders.com website.
Disclosures
None of the authors have any conflict of interest to declare.