Epileptic Disorders
MENUControl groups in paediatric epilepsy research: do first-degree cousins show familial effects? Volume 19, numéro 1, March 2017
Studies investigating the neurobehavioural comorbidities of paediatric epilepsy have collectively used a variety of control groups that include siblings or other family members, friends, persons with other chronic medical conditions, or individuals from the general population. Approaches to selecting a particular control group vary with the intent and design, as well as the constraints of each individual study. Furthermore, each potential choice of control group is associated with a range of unique advantages and disadvantages.
Sibling controls have been a commonly used control group in studies of behavioural, cognitive, and neurodevelopmental comorbidities in paediatric epilepsy (Bourgeois et al., 1983; Austin et al., 2001, 2002, 2011; Berg et al., 2008; Fastenau et al., 2009; Benn et al., 2010; Dunn et al., 2010; Ong et al., 2010; Sogawa et al., 2010; Bennett-Back et al., 2011; Hesdorffer et al., 2012; Smith et al., 2012; Zelko et al., 2014). One advantage of siblings as controls is that they allow for better control of unmeasured or unknown family-level environmental factors (Austin et al., 2002; Riva et al., 2002; Benn et al., 2010), given that they share similar sociocultural backgrounds and family experiences. Use of a sibling control group, therefore, can provide an advantage by controlling for differences in environmental experiences that might contribute to cognitive, psychiatric, or behavioural comorbidities. In addition, siblings may provide partial control for genetic factors which may assist in discriminating between disorder-specific sequelae and comorbidities attributed to a genetic predisposition related to the disorder of interest. Research designs that use sibling controls also provide practical benefits for recruitment and retention, as siblings commonly live in the same house (or in geographically nearby areas) and are therefore easier to recruit (Gauderman et al., 1999; Witte et al., 1999). Siblings also tend to be more invested in study goals and outcomes than controls from the general population, making them more motivated to participate and maintain longitudinal participation (Gauderman et al., 1999). These practical advantages make the use of sibling control groups an appealing control group.
Despite these benefits, the close genetic relationship between siblings can be problematic for interpreting results of cognitive and behavioural comorbidities, as well as neuroimaging anomalies, particularly when the primary disorder under investigation (i.e. childhood epilepsies) may have genetic associations with the behaviours under investigation, thereby making “unaffected siblings” a potentially less than optimal or biased control group. When cases have disorders with genetic correlates, and also have a large amount of shared genetic variance with controls, this can reduce bias but requires larger sample sizes to delineate the genetic contributions to the disorder under examination. In such cases, the effect of genetic differences may be less easily identified due to fewer genetic differences between participants and controls, therefore, siblings may mask true genetic effects that might otherwise be revealed using a control group that is more genetically distant (Heins et al., 2011). Consistent with these concerns, past studies have shown that unaffected siblings of those with epilepsy tend to be more similar on a number of neurocognitive, behavioural, and neuroimaging measures compared to general population controls (Singhi et al., 1992; Iqbal et al., 2009, 2015; Wandschneider et al., 2010, 2014; Aronu and Iloeje, 2011; Hesdorffer et al., 2012; Badawy et al., 2013; Tsai et al., 2013; Verrotti et al., 2013; Chowdhury et al., 2014; Alhusaini et al., 2015).
The general population, on the other hand, is usually considered ideal as a potential control group across different areas of scientific research (Ho et al., 2008). Using a sample from the general population as a control group provides for more genetic variability in the study sample, but may also lead to confounding effects due to genetic admixture. The concern is that a putative correlate of disease status may not be a result of the disease at all, but rather may arise owing to unmeasured population variation in genes that influence both disease occurrence and the manifestation of the putative correlate. While considered ideal for most research, general population controls may be more difficult to recruit compared to siblings, making the recruitment process more time and cost intensive (Gauderman et al., 1999). Additionally, as individuals from the general population may be more likely to have differing life experiences and sociocultural backgrounds, general population controls may provide less protection from environmental and demographic confounds compared to siblings (Gauderman et al., 1999; Riva et al., 2002; Gur et al., 2015). Lastly, and most importantly, general population controls may be less motivated to maintain participation in research studies compared to siblings (Gauderman et al., 1999), making longitudinal retention rates lower, reducing the study's statistical power and possibly introducing selection bias.
First-degree cousin controls provide an alternative to siblings and population-based controls. Cousins have been used in a variety of fields but not frequently employed in epilepsy research. In genetics and child development research, family-based association studies use cousins to reduce sociocultural and genetic variance and confounding (Geronimus et al., 1994; Gauderman et al., 1999; Witte et al., 1999; Turley, 2003), and utilization of such family-based designs has extended into diverse fields including schizophrenia, ADHD, and behavioural and social psychology research (McIntosh et al., 2005; Gur et al., 2007, 2015; D’Onofrio et al., 2010; Ljung et al., 2013; Larsson et al., 2014; Skoglund et al., 2014; Giordano et al., 2015; Kendler et al., 2015). Cardy et al. (2007) used a pure cousin control group to examine risk of a congenital disorder, while Riva et al. (2002) used a mixed cousin and sibling control group to study the effect of a surgical cancer treatment on cognitive functioning in a paediatric population.
Using cousins as controls represents one potential approach to combine the practical advantages of sibling controls with the strengths of general population controls. In fact, some studies (Skoglund et al., 2014; Giordano et al., 2015) have shown that general population controls share more similarities with cousins than siblings in terms of psychiatric outcomes, suggesting that cousins are genetically distant enough from participants to serve as a substitute for general population controls. Additionally, there is typically a larger pool of cousins available for recruitment and they are easier to age match than siblings (Gauderman et al., 1999; Witte et al., 1999), making recruitment of cousins more efficient for control groups.
Cousin controls may have other advantages over general population controls, as longitudinal studies require multiple follow-up assessments and retention of a related control group is usually better compared to unrelated controls (Gauderman et al., 1999). Similar to siblings, cousin controls tend to be more invested in the research given their close connection with an affected family member (Gauderman et al., 1999) with stronger incentive to participate in the study and cooperate with study tasks; all serving to reduce the amount of time, effort, and financial resources needed to enrol and retain participants in the investigation (Gauderman et al., 1999).
The purpose of the current study was to evaluate potential biases associated with use of first-degree cousin controls in research examining cognitive, psychiatric, and neuroimaging comorbidities of paediatric epilepsy. As noted, previous research has shown that unaffected siblings may share cognitive, behavioural or imaging “anomalies” with their affected siblings, manifesting as correlations between case and sibling controls. That is, there is greater relatedness between patients with epilepsy and unaffected siblings compared to population-based controls. Examination of the relatedness of children with epilepsy to first-degree cousins would similarly reveal any bias associated with a cousin control group. In the current study, we examined whether outcomes of children with recent-onset epilepsy predict those of their healthy cousins across a wide range of neurocognitive, behavioural, and neurobiological (i.e. MRI) measures. Our primary assumption is that the degree of bias in a control group, such as first-degree cousins, will be reflected in the number and strength of associations between the experimental and control group across dependent measures of interest. The more correlations observed between epilepsy and cousin controls, the more biased the control group; the fewer and weaker the associations between groups, the more the controls approach the status of an unbiased comparison group.
Methods
Participants
We recruited children with recent and new-onset epilepsy and their healthy first-degree cousin controls, aged 8 to 18 years, from paediatric neurology clinics at three Midwestern Medical Centers (University of Wisconsin-Madison, Marshfield Clinic, Dean Clinic). The following inclusion criteria were used for children with epilepsy:
- –a diagnosis of epilepsy within the past 12 months;
- –no other developmental disabilities or neurological disorders;
- –normal neurological examination;
- –normal neuroimaging (magnetic resonance imaging or computed tomography) results.
Prior to recruitment, the medical records for all patients were independently reviewed by a board-certified paediatric neurologist to confirm that participants had epilepsy, to provide confirmation of syndrome diagnosis, and to ensure that all other study criteria (e.g. normal neuroimaging) were met. Specific syndromes were classified using the modified diagnostic criteria of the International League Against Epilepsy Task Force on Classification and Terminology (Berg et al., 2010).
Participants in the control group were first-degree cousins and met the following inclusion criteria:
- –no history of seizures;
- –no early initial precipitating injuries (e.g. febrile convulsions);
- –no developmental or neurological disease;
- –no history of loss of consciousness greater than five minutes.
To obtain first-degree cousin controls, the mother of each participating child with epilepsy was asked to identify associated families with a potential first-degree cousin with epilepsy who met the standard inclusion and exclusion criteria. Potential candidate control families were then contacted by the study investigator who confirmed study requirements. More details regarding subject selection criteria have been published previously by our group (Hermann et al., 2006).
Procedures
This study was reviewed and approved by the Institutional Review Boards of all participating institutions. Families and children gave informed consent and assent on their study participation day. All procedures were consistent with the Declaration of Helsinki (World Medical Association Declaration of Helsinki, 1991). The children subsequently underwent neuropsychological testing and an MRI scan, and parents completed the Child Behaviour Checklist (CBCL).
Neuropsychological assessment
Participants received comprehensive neuropsychological testing using a test battery that included standardized measures of intelligence, academic achievement, executive function, processing speed, and memory. Test selection was based on domains of interest and applicability to a broad age range to ensure the same test versions could be administered to children of varying ages (table 1). A parent-completed measure for emotional and behavioural problems was also included and is listed in table 1.
MRI acquisition
Images were obtained on a 1.5T GE Signa MRI scanner (GE Healthcare, Waukesha, WI, USA). Sequence acquired for each participant was a T1-weighted, three-dimensional (3D) spoiled gradient recall (SPGR) using the following parameters: TE=5 ms, TR=24 ms, flip angle=40 degrees, NEX=1, slice thickness=1.5 mm, slices=124, plane=coronal, field of view (FOV)=200 mm, and matrix=256 × 256. All MR images were inspected before image processing. Image quality was rated on a 5-point (0-4) scale and we required a minimum quality of 3 for the scan to be included in this analysis.
The reported volumetric data were produced using Freesurfer 5.3. Post-processed data were visually inspected, and manual interventions were used to correct surface errors. In addition to the standard Freesurfer processing stream, we also extracted the cortical grey matter surface volume using a larger lobar parcellation scheme.
Analytic plan
For this analysis, we included only children with epilepsy with at least one related cousin in the study (n=37) and all enrolled cousins (n=100). Cognitive and behavioural scores used in this analysis are specified in table 1, and quantitative MRI measures used are listed in table 2.
Population controls would be expected to differ on average from epilepsy (case) participants on many of the measures, but the concern of interest here is the extent to which cousin controls will differ from population controls owing to their genetic, socio-cultural, and environmental proximity to the cases. To test this, we exploited the variability in the association of the foregoing measures in the cases. The logic was as follows. If the cousins tend to be a biased sample, from the general population perspective on these measures based on having a cousin with epilepsy (or proband), then, within our sample, we would expect the control cousins of more impaired cases to perform worse and for those of less impaired cases to perform better. That is, we expect the cousin control measures to be positively correlated with the epilepsy case measures.
To test the degree to which this is true, the cousin measures were regressed on their corresponding epilepsy case measures using a linear mixed model (Laird and Ware, 1982).
In this model, for a given measure, the cousin control and epilepsy case measures are given by Cousij and Epij, Ui is the nuclear family-level random effect, and ∈ij is the residual error. The family random effect accounts for the fact that, in our design, more than one cousin control could be linked to a given case; multiple cousins are often siblings and may therefore correlate with one another. In the model, the variance of the cousin measures is β2* VarEpi + VarU + Var∈, and the covariance between the control and case measures is β * VarEpi. The intercept β0 accounts for the fact that the mean control response is on average better than the mean case response.
For each measure, analyses included computing the empirical mean and variance of both the epilepsy cases and cousin controls. The mixed effects model was then fitted to the data; the fitted model-based variance of the cousins was compared to the empirical variance to ensure a close match (which always occurred). Using the fitted model, the correlation between the cases and their cousin controls was computed and tabulated, and the p values for β = 0 were obtained. Using aggregate analyses, the distribution of p values was compared across all measures to the uniform (0,1) distribution that would be expected if there were no association in the measures between cases and controls. We also examined the distribution of correlation values for the degree to which they were centred on zero. Mixed models were estimated using PROC MIXED and aggregate summaries were constructed using PROC UNIVARIATE and PROC SGPLOT in SAS version 9.4 (Cary, NC).
Results
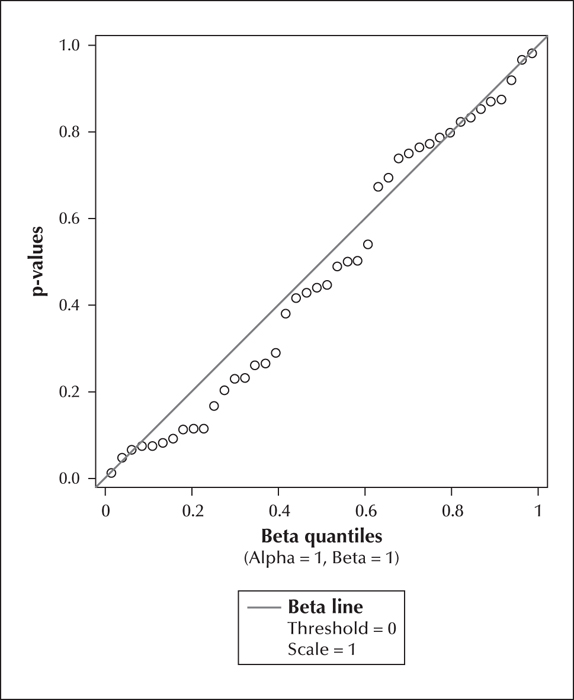
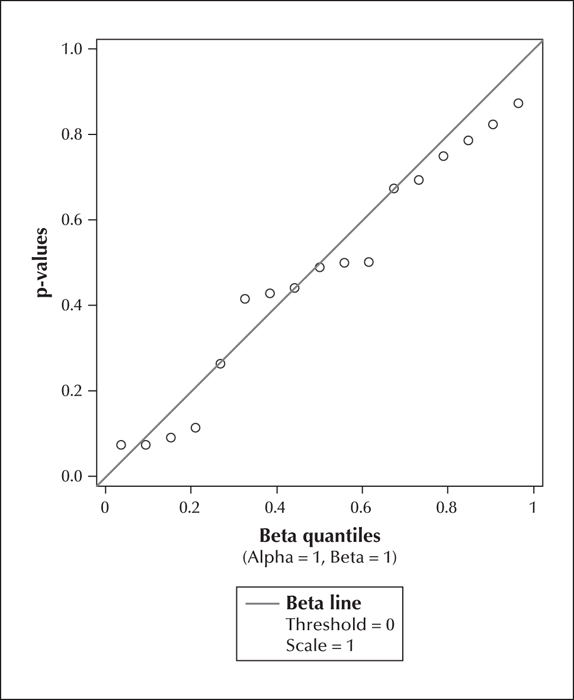
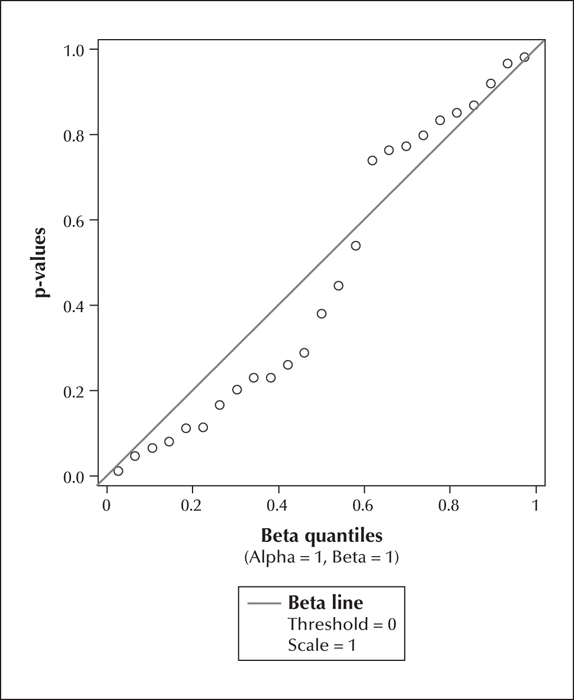
Table 3 provides information regarding the epilepsy and cousin control participants. We fitted the model to the 42 distinct measures (13 cognitive, 4 behavioural, and 25 imaging). Table 1 and table 2 provide descriptive statistics and correlation coefficients for the combined 17 cognitive and behavioural scores and the 25 brain volume measurements, respectively. All correlations ranged between -0.28 to 0.43 (median: 0.06). P values obtained from the mixed models were plotted against a Uniform (0, 1) distribution (figure 1). The p values were found to be concentrated near the 45-degree diagonal line, which means their distribution was close to the uniform (0, 1) distribution which would be expected under the null hypothesis of no association. Out of the 42 p values, only two were p values deviated from the Uniform (0,1) was not significant (KS=0.12: p>0.25). Associations were also examined as a function of domain of interest (cognition and behaviour vs. brain measurements). Figures 2 and 3 show p values plotted against a Uniform (0, 1) distribution for cognitive and behavioural (figure 2) and imaging (figure 3) measures. Again, there was no overall significant association with the domain-specific measurements (KS for cognition and behaviour=0.15, p>0.25; KS for brain measurements=0.19, p>0.25). The correlations ranged between -0.17 to 0.24 (median: 0.08) for the cognition and behaviour measures, and -0.28 to 0.43 (median: 0.05) for the brain measurements.
Taken across the 42 measures, and within each of the sub-domains, the minimal association between cousins and cases suggests no selection bias in the cousin group relative to the general population.
Discussion
The results of this study reveal minimal correlative relationships between children with epilepsy and their unaffected first-degree cousins across a diverse array of neuropsychological, behavioural, and neuroimaging measures, even after accounting for differences due to having epilepsy versus unaffected by epilepsy. The landscape of dependent measures was relatively broad, with the neuropsychological measures representing classic domains of cognitive ability (intelligence, language, learning and memory, executive function, and processing speed), the behavioural measures assessing broad and commonly used measures of externalizing and internalizing disorders and social competence, and the neuroimaging measures examining cortical, subcortical, and cerebellar volumes. This lack of association between the epilepsy participants and cousin controls suggests a lack of selection bias in the latter, which is especially important as some epilepsies with genetic contributions were included. The obtained pattern of results is the pattern that would be expected if epilepsy participants were compared to population based controls, although such comparisons were not possible in the current study.
In the current study, participants with epilepsy were not required to supply a cousin control in order to meet study inclusion criteria. Whereas this protects against introducing a sampling bias in the patient group, it does present a possible sampling bias threat in the cousin control group. Thus, our cousin control group may not be representative of all cousins of children with epilepsy. Additionally, we were not able to directly examine the relationship between cousin controls and general population controls on any of the measures collected in this study. Such a comparison would provide insight into the validity of substituting cousins for general population controls in epilepsy research and should be considered in future research. Additionally, our behavioural measures were limited in scope compared to the wide range of cognitive and quantitative MRI variables, however, it is important to note that correlations on these measures were not significant. The current study provides preliminary evidence that use of first-degree cousins as controls is defensible. Given the associated advantages associated with this group, it may be worthwhile to consider recruiting first-degree cousins in medical and neurological research.
Supplementary data
Summary didactic slides are available on the www.epilepticdisorders.com website.
Acknowledgements and disclosures
This study was supported by NINDS 3RO1-44351.
None of the authors have any conflict of interest to disclose.