European Journal of Dermatology
MENUPrevention of non-melanoma skin cancers with nicotinamide in transplant recipients: a case-control study Volume 27, numéro 4, July-August 2017
Nicotinamide is the water-soluble, amid form of vitamin B3 (niacin) and the precursor of nicotinamide adenine dinucleotide (NAD+), an essential cofactor for adenosine triphosphate (ATP) production. Other forms of vitamin B3 include niacin (nicotinic acid), more complex amides, and a variety of esters, such as inositol hexanicotinate.
The main dietary sources of nicotinamide and niacin are various meats, dairy products, legumes, beans, nuts, green leafy vegetables, cereals, coffee, and tea. The recommended daily intake of vitamin B3 in niacin equivalents is 16 mg for men, 14 mg for women, 18 mg for pregnant women, and 17 mg for lactating women. A lack of this vitamin can cause pellagra, a disease characterized by the triad of dementia, dermatitis, and diarrhoea [1]. Nicotinamide has a long heritage of use in dermatology. Recently, interest in its use has focused on its chemopreventative role in non-melanoma skin cancers (NMSCs). More specifically, it has recently been reported to be effective in reducing the rates of NMSCs and actinic keratosis (AKs) in high-risk patients, such as those who previously had histologically confirmed NMSCs [2-4].
AK is a photo-induced skin diseasewhich can progress into invasive squamous-cell carcinoma (SCC). In the absence of reliable predictors of development of SCC, AK treatment is highly recommended. Lesion-directed cryosurgery, field-directed therapy with 5-fluorouracil, imiquimod, diclofenac, and ingenol mebutate are commonly used to treat AKs, although they may cause skin infections, scarring, and discomfort or pain [5].
The objective of our study was to test the efficacy of oral nicotinamide in preventing and treating AKs in transplant recipients.
Materials and methods
Between January and July 2015, all transplanted patients attending the Dermatologic Clinic of the University of Genoa for a skin check were examined over the entire body for detection of AKs. Patients presenting at least one AK (that had not been previously treated by medical or surgical treatments) were included in the study, after written informed consent. Immunosuppressive drugs that patients took to prevent rejection of the transplanted organ were recorded. AKs were identified, measured (cm2), and photographed for follow-up and, if patients agreed, biopsied for histopathology. The AK size (cm2) for every patient was determined in order to obtain a single datum for each patient, referred to as the “keratotic area.” This allowed us to evaluate the overall effect nicotinamide played on the individual. In the keratotic area, the biopsied lesions were not considered because of the possibility of variations due to tissue removal and the resulting inflammation and scarring. The recruited patients who had keratotic areas of comparable size were assigned to two groups. Group 1 took nicotinamide, 250 mg thrice daily, (cases) and Group 2 did not take any drug to treat AKs (controls). For each case, one matching control was selected without randomization. The total area of AK was calculated for the group of cases and group of controls.
During the period of follow-up, other medical and surgical treatments for AKs were avoided for both cases and controls. All patients were strongly recommended to use the same sunscreen with a sun protection factor (SPF) of 50+ every day for sun-exposed skin areas. At baseline and after 15 and 30 days from the start of nicotinamide treatment, blood level of the immunosuppressant drugs was assessed to exclude any interference by nicotinamide. After six months, all patients were re-evaluated; AKs were re-identified, measured, photographed and, in the five patients who previously underwent an AK biopsy, a new biopsy was performed for histopathology.
Statistical analyses were performed using the Student t test.
Results
We recruited 38 transplanted (eight liver and 30 kidney) patients with skin phototype II (10 patients), III (19 patients), or IV (nine patients) (Fitzpatrick classification) with single or multiple AKs, both atrophic and hypertrophic types. Nineteen patients were assigned to Group 1 (cases) and 19 other patients to Group 2 (controls). The average time from transplantation was 8.3 years (range: 0-36 years). To prevent transplant rejection, 31 patients were treated with cyclosporine A (13 mg/kg/daily) while seven patients were treated with tacrolimus (0.5 mg/kg/daily). Five patients of Group 1 underwent an AK biopsy for histopathology at baseline and after six months. At baseline, the mean values of the keratotic area were 0.6033 ± 1.1838 cm2 in Group 1 and 0.6745 ± 0.6851 cm2 in Group 2; no statistically significant difference was observed between AK size of the two groups (p>0.05). Histopathology confirmed the clinical diagnosis of AKs in all the five biopsied lesions (three hypertrophic and two atrophic type AKs). AK biopsies were performed on Group 1 only in order to show whether, and how, the treatment with nicotinamide affected AKs.
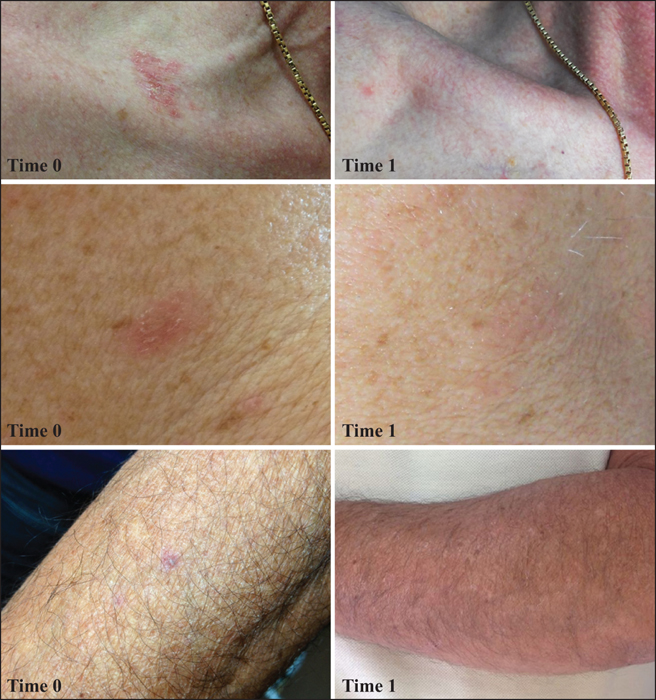
After six months, among the cases, AKs of both atrophic and hypertrophic types had significantly decreased in size in 18 of the 19 patients (88%). Among these 18 patients, seven patients (42%) had shown complete clinical regression (figure 1). Skin biopsies performed at the end of the study, close to the same sites where the first bioptic samples had been taken six months before, showed normal skin. No patient in Group 1 developed new AKs. Only one patient discontinued nicotinamide because of diarrhoea. There were no other adverse effects and no patients dropped out of the study or missed appointments.
Conversely, among the controls, 91% showed an increase in AK size and/or developed new AKs. Moreover, seven pre-existing AKs progressed to SCC. At this time, the mean values of the keratotic area were 0.1067 ± 0.13 cm2 in Group 1 and 1.01 ± 0.9295 cm2 in Group 2; the difference was statistically significant (p=0.010) (table 1). The seven new SCCs in the control group were surgically removed and then histologically confirmed. Other skin cancers (e.g. basal cell carcinomas) during the study period were not been observed.
The blood levels of the immunosuppressive drugs after 15 and 30 days from the start of nicotinamide treatment were comparable to the levels at the beginning of the study, remaining within the normal ranges from the beginning to the end of the study (cyclosporine A: 500-700 ng/mL for liver transplantation and 600-900 for kidney transplantation; tacrolimus: 5-10 ng/mL for both liver and kidney transplantation).
Discussion
In the general population, UV radiation is the main cause of skin cancers, the most common malignancies in Caucasians [6]. UV radiation is responsible for gene mutations and also plays a role in carcinogenesis by suppressing cutaneous immune responses to tumour antigens [7]. Organ transplant recipients (OTR), who chronically receive immunosuppressive treatment to maintain adequate graft function, are at increased risk of developing many types of skin cancers, including SCC, Merkel cell carcinoma, and Kaposi's sarcoma. The spectrum of skin cancers in OTR and their prevalence depends on the grafted organ, the type and duration of immunosuppressive therapy, UV radiation exposure, genetic factors, and immunological control of oncogenic viruses which are involved in the pathogenesis of skin neoplasms.
In OTR, the most common skin cancer is SCC which occurs up to 250 times more frequently than in the general population. Within 20 years of transplantation, 20-75% of OTR are affected by at least one SCC, especially in UV light-exposed body regions [8, 9]. Therefore, early recognition and treatment of precancerous lesions are recommended to prevent the development of invasive tumours. Moreover, as sunscreens provide only partial protection from UV-induced immunosuppression [10], other approaches are needed to obtain major protection from skin cancer.
Nicotinamide is an inexpensive and non-toxic agent, which has been used clinically for the treatment of several inflammatory skin disorders, including autoimmune blistering disorders, acne, rosacea, and atopic dermatitis [3, 11]. It has also been shown to prevent UV-induced immunosuppression and carcinogenesis in mice [12] and protects from UV immunosuppression in humans [13]. Previous studies suggested that topical nicotinamide enhances the resolution of AKs in heavily sun-damaged individuals [14], although its effects on proliferative markers in these lesions remain unknown.
The mechanisms through which nicotinamide is effective in preventing and treating AKs are not yet completely understood.
Nicotinamide is the precursor of NAD+ and the sole substrate and inhibitor of the nuclear enzyme poly-ADP-ribose polymerase (PARP-1), which has central functions in DNA repair and genomic stability [15, 16]. When PARP-1 is over-expressed, however, NAD+ may be over-consumed, leading to cellular dysfunction or necrosis. Irradiation with UVB is known to trigger NAD+ depletion in both in vivo murine epidermis, as well as cultured human cells [17]. Nicotinamide deficiency in human cells has been shown to alter the expression of p53, induce genomic instability, and reduce survival following exposure to solar-simulated UV radiation (containing both UVB at 290-320 nm and UVA at 320-400 nm) [17]. In animal studies, nicotinamide deficiency also caused changes in NAD+, increasing genomic instability, impairing cellular responses to DNA damage, and increasing cancer incidence [18, 19].
On the contrary, nicotinamide supplementation boosts cellular energy and may enhance energy-dependent cellular processes, such as DNA repair [15].
Recently, several authors have focused their attention on the efficacy of nicotinamide in preventing and treating AKs. Indeed, AKs are extremely common skin lesions (over 40% of Australian adults have at least one lesion) and it is estimated that 0.6% progress to SCC after one year and 3% after four years. In a phase II double-blinded controlled trial, 34 otherwise healthy patients were randomized to receive oral nicotinamide at 500 mg or matched placebo twice daily for four months. In the nicotinamide group, a 35% reduction in total AK, relative to the placebo group, was found at four months (p = 0.0006) [2]. Chen et al. recently reported that in 386 immunocompetent patients at high risk of having skin cancer, nicotinamide, 500 mg twice daily, reduced new NMSCs by 23% (p = 0.02), with 20% fewer basal cell carcinomas and 30% fewer SCCs, compared with placebo. AKs were also significantly reduced by nicotinamide in the same phase III randomized controlled trial [3].
The efficacy of nicotinamide in preventing NMSCs and its safety in OTR were first evaluated by our group in a study that involved 24 transplant recipients. Patients were randomly divided into two groups; Group 1 took nicotinamide (250 mg thrice/daily) and Group 2 were without nicotinamide. After six months of follow-up, AKs were reduced in size in 88% of the patients who took nicotinamide; of these patients, 44% had a complete clinical remission. In contrast, in 91% of controls, the size of AKs increased and/or new lesions developed [20].
Subsequently, Chen et al. reported a phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients [21]. AK counts were not significantly lower in participants receiving nicotinamide (500 mg twice daily) compared with placebo, with greater chemopreventative efficacy of nicotinamide seen in participants with a higher number of previous NMSC. The results obtained in our study differ slightly from those of Chen et al. [21] and are more encouraging. This may be due to several differences between the two OTR populations studied. First, the type and dosages of immunosuppressive drugs in OTR may account for the difference; a higher level of immunosuppression can cause nicotinamide to be less efficient in preventing and treating AKs. Second, unfortunately, Chen et al. did not report the type of immunosuppressive drugs nor the dosages used by their patients [21].
In conclusion, oral nicotinamide may be a promising alternative for the prevention and treatment of skin cancers, especially in high-risk populations, such as OTR. Benefits include the double action of preventing and treating AKs, non-invasiveness, non-toxicity, poor side effects, and low cost. Further studies with a larger sample of OTR patients and a longer follow-up period are needed to further support our conclusion.
Disclosure
Financial support: none. Conflict of interest: none.