Hépato-Gastro & Oncologie Digestive
MENUHepatotoxicity of antidepressant drugs Volume 24, issue 4, Avril 2017
Hôpital Antoine Béclère, service d’hépato-gastroentérologie et nutrition,
157 rue de la Porte de Trivaux,
92141 Clamart cedex,
France
Univ Paris-Sud,
Faculté de Médecine Paris-Sud,
Le Kremlin-Bicêtre F-94276,
France
Hôpital de Bicêtre,
service de psychiatrie,
Le Kremlin Bicêtre,
F-94276,
France
- Key words: drug-induced liver injury, antidepressants, side effect
- DOI : 10.1684/hpg.2017.1433
- Page(s) : 357-66
- Published in: 2017
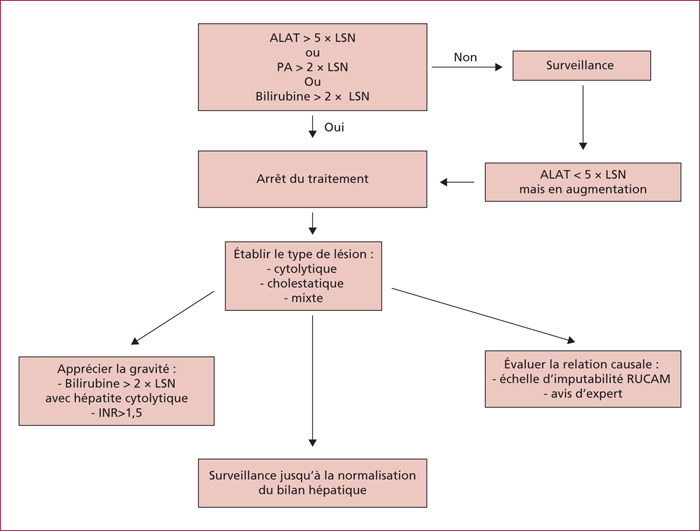
Mild abnormal liver function is detected in 0.5%-3% of patients treated with antidepressants, and the incidence of drug-induced liver injury related to antidepressants is of 1.28-4 cases per 100,000 patient-years. Antidepressant liver toxicity is underestimated due to case under-declaration and absence of precise diagnostic criteria. The risk factors for antidepressant-induced liver injury are poorly known. Drug-drug interactions or a cross-toxicity for tricyclic antidepressants due to their similar tricyclic structure, have been described. A dose-response relationship was reported for duloxetine, nefazodone and mianserin. Liver toxicity due duloxetine seems to be more frequent in patients with underlying chronic liver disease. Temporal relationship between drug prescription and hepatotoxicity, as well as evolution of liver function tests upon drug discontinuation is essential diagnostic criteria. Liver damage is often of the hepatocellular type and less frequently of the cholestatic and mixed types. Generally, liver function tests rapidly normalize with drug withdrawal, but cases of fulminant hepatic failure have been described. The drugs for which the frequency of reported hepatic toxicity appears to be highest are monoamine oxidase inhibitors, tricyclic antidepressants, nefazodone, bupropion, duloxetine and agomelatine. Citalopram, escitalopram, paroxetine and fluvoxamine seem to have lower hepatotoxicity. Prompt discontinuation of the drug responsible and surveillance of liver function tests are essential.