Epileptic Disorders
MENUOxcarbazepine-induced myoclonic status epilepticus in juvenile myoclonic epilepsy Volume 15, issue 2, June 2013
epd.2013.0563
Auteur(s) : Martina Fanella1, Gabriella Egeo1,2, Jinane Fattouch1, Sara Casciato1, Leonardo Lapenta1, Alessandra Morano1, Anna Teresa Giallonardo1, Carlo Di Bonaventura1 c_dibonaventura@yahoo.it
1 Department of Neurology and Psychiatry, Epilepsy Unit, Sapienza University of Rome
2 San Raffaele Pisana IRCCS, Rome, Italy
Correspondence.Carlo Di Bonaventura Department of Neurological Sciences, Policlinico Umberto I, Sapienza University of Rome, Viale dell’Università 30, 00185-Rome, Italy
Since the first antiepileptic drugs (AEDs) were introduced, clinical experience has documented the occurrence of seizure worsening associated with the use of inappropriate therapy. AED-related seizure induction is a rare condition that was first recognised as recently as the early 1990s and should be differentiated from seizure occurrence based on the natural course of the epilepsy. Such seizure worsening, the occurrence of which is closely related to the introduction of a specific AED, consists of an increase in both seizure frequency/severity and the onset of new seizure types. The reversibility of the clinical picture through drug withdrawal is typical and usually supports the diagnostic hypothesis. The majority of the cases described in the literature concern the precipitation or exacerbation of generalised seizures (absence, myoclonic, and atonic seizures) in patients with idiopathic generalised epilepsy (IGE) who are administered carbamazepine (CBZ), phenytoin (PHT) (Genton et al., 2000; Osorio et al., 2000) or vigabatrin (VGB). IGEs, particularly juvenile myoclonic epilepsy (JME), comprise the types of epilepsy most frequently associated with drug-induced worsening of seizures (Perucca et al., 1998). JME is a common form of IGE with typical clinical and EEG features. The gold standard treatment is valproic acid monotherapy, which controls seizures in 82-97% of cases (Genton et al., 1994). JME may be misdiagnosed and treated as a partial epilepsy. In this report, we describe a case of myoclonic status epilepticus (MSE) recorded by video-EEG in a patient with JME treated with oxcarbazepine (OXC).
Case study
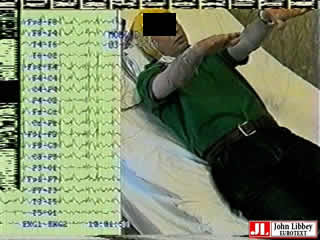
We describe a 44-year-old male affected by JME. He had a positive family history and his father and two cousins on his father's side were affected by an ill-defined epilepsy. His seizures began at the age of 13 with myoclonic jerks which predominantly involved the upper limbs and oro-facial muscles, and usually occurred upon awakening. On only two occasions before the diagnosis, both after sleep deprivation, the myoclonic jerks were followed by a generalised tonic-clonic seizure. Previous EEG recordings showed a typical pattern consisting of spontaneous generalised polyspike-and-wave discharges generalised polyspike-and-wave discharges and a photoparoxysmal response to intermittent photic stimulation (IPS). Complete seizure control was achieved as soon as phenobarbital was introduced to the patient's therapy. When the therapy was suspended after one year, the seizures reappeared and a generalised tonic-clonic event occurred. Therapy consisting of clonazepam at a daily dose of 2 mg was reintroduced. This drug did not, however, completely control the seizures, which were characterised by myoclonic jerks associated with a subjective manifestation described as “something in the stomach…”, often followed by difficulty in speaking; “I can’t speak well…”. Since this ictal semiology suggested the presence of partial epilepsy, therapy with OXC at 900 mg per day was started. Approximately four weeks after OXC was introduced, the patient was admitted to our clinic due to dramatic worsening of his clinical condition; he presented with widespread, asynchronous and asymmetric myoclonic jerks, affecting different parts of the body with a predominant involvement of the upper limbs and oro-pharingeal muscles, associated with a subjective sensation, described as “I feel confused… I can’t speak well…”. Myoclonus involving the oro-pharingeal muscles resulted in a language disturbance consisting of a slowing-down and fragmentation of speech. Video-EEG monitoring, including polygraphy, documented a MSE with positive and negative myoclonus, correlating with repetitive, continuous, rhythmic generalised polyspike-and-wave discharges (figures 1 and 2).
The patient was treated with 4 mg iv lorazepam, which promptly led to the resolution of the myoclonic phenomena and EEG abnormalities. After the MSE resolved, OXC therapy was discontinued and levetiracetam, at a daily dose of 2,000 mg, was introduced. Two years after the initiation of levetiracetam therapy, the patient is currently seizure-free, which was confirmed by normal EEG and response to IPS.
Discussion
Epileptic generalised syndromes are known to be aggravated by the inappropriate use of some AEDs, including CBZ, PHT, VGB, as well as other molecules that may exacerbate pre-existing seizures or induce the onset of various ictal phenomena (Perucca et al., 1998; Thomas et al., 2006). A worsening of seizures has recently been reported following the use of OXC in some generalised idiopathic epilepsies, including JME (Gelisse et al., 2004). The dramatic exacerbation of the myoclonic seizures witnessed in our case was clearly related to OXC, as is suggested by the close temporal relationship between the introduction of OXC and the worsening of the seizures, as well as between the tapering of OXC and the improvement in the patient's clinical condition. This reported case is of considerable interest for several reasons. Since MSE is a very rare event in JME, the data in the literature on this condition are limited to sporadic cases. The most important triggering factors are: excessive alcohol intake, sleep deprivation, poor patient compliance, and inadequate AEDs. This is, to our knowledge, the first report of a clearly documented video-EEG of MSE induced by OXC in a patient with JME. A case of an absence status with a continuous and fixed epileptic condition has previously been reported following the use of the same drug in juvenile absence epilepsy (Gelisse et al., 2004). Although CBZ- and PHT-related exacerbation of myoclonic seizures in IGE is well known (Genton et al., 2000; Osorio et al., 2000), seizure worsening following treatment with OXC has been observed in only seven cases, four of which were cases of JME (Gelisse et al., 2004). Seizure worsening, consisting of increased seizure frequency, a renewed onset of absences, and myoclonic seizures associated with behavioural and EEG changes has also been documented in paediatric patients with epilepsy treated with OXC monotherapy (Chapman et al., 2003; Grosso et al., 2006; Kaddurah and Holmes, 2006; Vendrame et al., 2007; Menon et al., 2011; Veerapandiyan et al., 2012).
In addition to presenting this case study, we have reviewed the literature of reports that describe OXC-induced worsening of seizures, the electroclinical characteristics of which are summarised in table 1.
Table 1 Electroclinical characteristics of patients in the literature with OXC-induced worsening of seizures.
| No. of cases | Sex/age at onset | Sz type before OXC | Epileptic syndrome | EEG before OXC | OXC daily dose | Concomitant AEDs | Worsening Sz with OXC | New Sz type with OXC | EEG with OXC | Outcome without OXC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chapman et al., 2003 | 1 | F/7 | SPS +/- Sec GTCS Todd's paralysis | BECTS | Left CT BFEDC | N/A | No | No | Atypical absences Eyelid fluttering |
Interictal: left CT BFEDC, diffuse ED Ictal: left hemisphere ED |
Sz free |
| Gelisse et al., 2004 | 6 | F/10 | GTCS/MJ/A | JME | PPR; Left T SW | 1,200 mg/die | No | MJ/A | No | PPR, Interictal: GPSW | Sz free |
| M/6 | GTCS/MJ/A | CAE vs JME | N/A | 600 mg/die | No | GTCS/MJ | No | N/A | ↓Sz freq | ||
| F/13 | GTCS/MJ | JME | N/A | 1,200 mg/die | VPA/PB/CNZ | GTCS/MJ | No | N/A | ↓Sz freq | ||
| F/12 | GTCS/MJ | JME | N/A | 1,200 mg/die | No | GTCS/MJ | A | N/A | ↓Sz freq | ||
| F/12 | GTCS/MJ/A | JAE | GS&W | 600 mg/die | No | MJ/A | No | Ictal: GPSW | Sz free | ||
| F/19 | GTCS | IGE | PPR | 300 mg/die | No | No | MJ | N/A | ↓Sz freq | ||
| Kaddurah and Holmes, 2006 | 2 | F/14,5 | GTCS | GE and 22q del syndrome | Normal | 15 mg/kg/die | No | GTCS | MJ | PPR, irregular S&W | Sz free |
| M/1,5 | CPS + Sec GTCS | PE and genetic syndrome | Normal | 25 mg/kg/die | No | No | MJ | Interictal: GS&W | Sz free | ||
| Grosso et al., 2006 | 3 | M/4,8 | SPS +/- Sec GTCS | BECTS | Right CT diphasic S/S&W, left T slow S | 40 mg/kg/die | No | No | Atypical absences | Ictal: GS&W | Sz free |
| M/5,2 | SPS +/- Sec GTCS Todd's paralysis | BECTS | Right CT diphasic S/S&W | 42 mg/kg/die | No | No | Atypical absences Eyelid fluttering |
Interictal: right CT S&W, GS&W Ictal: diffuse ED |
Sz free | ||
| M/4,3 | SPS Todd's paralysis |
BECTS | Right CT S | 16 mg/kg/die | VPA | SPS | No | Interictal: right CT S&W | ↓Sz freq | ||
| Vendrame et al., 2007 | 12 | F/4 | CPS | PE | Normal | 20 mg/kg/die | No | No | GTCS | GPSW | ↓Sz freq. |
| F/3 | CPS | PE | Left F S and slow waves | 45 mg/kg/die | No | No | No | left S&W/GS&W | ↓Sz freq. | ||
| M/5 | CPS | PE | Normal | 22 mg/kg/die | No | No | No | GS&W | ↓Sz freq. | ||
| M/6 | CPS + Sec GTCS | PE | Normal | 24 mg/kg/die | No | No | MJ/A | GS&W | Sz free | ||
| M/4 | CPS + Sec GTCS | PE | Normal | 28 mg/kg/die | No | CPS + Sec GTCS | MJ | GPS | Sz free | ||
| M/15 | CPS + Sec GTCS | PE | Right F S | 20 mg/kg/die | No | No | No | Bil F S | Sz free | ||
| F/10 | GTCS | PE | Right parasagittal S&W | 24 mg/kg/die | No | GTCS | No | right parasagittal S&W/GS&W | ↓Sz freq. | ||
| M/4 | GTCS | PE | Left F S | 20 mg/kg/die | No | GTCS | No | GS&W | Sz free | ||
| M/8 | GTCS | PE | Right T slow waves/SW | 24 mg/kg/die | No | No | No | GPSW | N/A | ||
| F/10 | GTCS | PE?* | Normal | 24 mg/kg/die | No | No | No | GS&W | Sz free | ||
| F/15 | GTCS | PE?* | Normal | 17 mg/kg/die | No | GTCS | No | GPS | Sz free | ||
| M/9 | GTCS | PE?* | Bil slowing | 26 mg/kg/die | No | No | MJ/A | OIRDA | Sz free | ||
| Menon et al., 2011 | 1 | F/8 | GTCS/EMA | JS | N/A | 20 mg/kg/die | LGT | GTCS/EMA | MJ | PPR Interictal: GPS Ictal: GPSW |
↓Sz freq. |
| Veerapandiyan et al., 2012 | 1 | F/1,5 | CPS +/- Sec GTCS | PE | Interictal: right posterior waves, right CP SW/S,
left F SW/S Ictal: right T sharp rhythmic |
14 mg/kg/die | No | CPS+/- Sec GTCS | Spasms Todd's paralysis |
Interictal: diffuse hypsarrhythmia Ictal: generalised waves |
↓Sz freq. |
A: typical absences; EMA: eyelid myoclonia with absence; CPS: complex partial seizure; GTCS: generalised tonic-clonic seizure; MJ: myoclonic jerks; SPS: simple partial seizure; BECTS: benign epilepsy with centrotemporal spikes; CAE: childhood absence epilepsy; GE: generalised epilepsy; IGE: idiopathic generalised epilepsy (not further classified); JAE: juvenile absence epilepsy; JME: juvenile myoclonic epilepsy; JS: Jeavons syndrome; PE: partial epilepsy; BFEDC: benign focal epileptiform discharges of childhood; ED: epileptiform discharges; GPS: generalised polyspikes; GPSW: generalised polyspike wave; GS&W: generalised spike and wave; S: spike; S&W: spike and waves; SW: sharp waves; OIRDA: occipital intermittent rhythmic delta activity; PPR: photoparoxysmal response; AEDs: antiepileptic drugs; CNZ: clonazepam; LGT: lamotrigine; OXC: oxcarbazepine; VPA: valproate; PB: phenobarbital; CP: centro-parietal; CT: centro-temporal; F: frontal; T: temporal; Bil: bilateral; freq: frequency; Sec: secondary; Sz: seizure; N/A: not applicable.
* Syndrome classification is not well defined (according to authors; presumed but not confirmed PE).
Although the exact mechanism of action of OXC is unknown, a blockage of voltage-dependent sodium channels has been hypothesized. The active metabolite of OXC, a monohydroxy derivate, is responsible for the majority of therapeutic antiepileptic actions. As far as OXC is concerned, the mechanism underlying myoclonic seizure worsening is not yet fully understood, although an imbalance between cortical excitation and inhibition has been suggested. Indeed, similar to other AEDs that act on voltage-gated sodium channels, such as CBZ, a dysregulation of channel blockage or overblockage may also be implicated by the effects exerted by OXC; pharmacokinetic and experimental evidence regarding this issue is, however, still incomplete. Some experimental data derived from animal models of IGE demonstrate that OXC and CBZ share the ability to aggravate absence seizures by enhancing GABAA receptor action, particularly in specific brain regions such as the ventrobasal nucleus of the thalamus (VB) (Liu et al., 2006; Zheng et al., 2009). Thus, from a pathophysiological point of view, the facilitation or suppression of seizures depends on the balance between the inhibitory and excitatory activities of the thalamocortical network. The structure that plays a pivotal role in the activation of cortical sensorimotor areas (and consequently in the synchronisation of the epileptic discharge) within this network is the VB complex of the thalamus, which enhances GABAergic action. However, the facilitating activity of this nucleus is normally inhibited by the GABA-mediated activity of the reticular nucleus of the thalamus (Rt). Although OXC is presumed to enhance GABAA transmission of the VB nucleus, it does not have a similar effect on Rt. This different effect results in an imbalance in GABA transmission since the increase in VB activity is not sufficiently contrasted by the inhibitory action of Rt. This mechanism of action may explain the worsening of seizures. Another interesting, although hardly surprising, finding observed in this case is the coexistence of positive and negative myoclonic jerks. Negative myoclonus (NM) is a non-specific motor disorder that can occur in various neurological diseases. Epileptic NM is defined as the abrupt interruption of tonic muscle activity without prior positive myoclonia in the agonist-antagonist muscles. The cortical areas mediating NM are the primary motor cortex, premotor cortex, supplementary motor area, and primary somatosensory area (Rubboli and Tassinari, 2006). The mechanism through which CBZ and OXC determine epileptic NM is not clear. The afore-mentioned alteration in the balance between the inhibitory and excitatory circuits is considered to be the cause of prolonged postsynaptic inhibition. Consequently, treatment with CBZ or OXC in some epileptic subjects may induce localised or generalised epileptic activity and thus interfere with the cortical motor impulse, thereby determining an interruption of the continuous contraction of the muscle.
From a clinical point of view, a drug-induced increase in seizures is both a serious and common problem that is often neglected by medical staff. This phenomenon appears to be related to an incorrect diagnosis of the type of seizure or syndrome; indeed, misinterpretation of typical absences as focal seizures (especially as temporal lobe seizures) and of myoclonic seizures as focal clonic seizures is a relatively common error. In the clinical setting, “focal” features and “atypical” subjective manifestations (with or without asymmetric and lateralised EEG abnormalities) should be considered even in patients with JME (Jayalakshmi et al., 2010). Physicians should always carefully evaluate clinical and EEG data before administering AEDs in order to make a diagnosis as accurate as possible and consequently minimise the risks of drug-induced worsening of seizures.
Disclosures
None of the authors has any conflict of interests to disclose.
Legends for videosequences Video sequence Myoclonic status epilepticus documented by video-EEG, including polygraphy (bilateral extensor digitorum communis), showing subcontinuous positive and negative myoclonus involving the upper limbs. Key words for video research on www.epilepticdisorders.com Syndrome: juvenile myoclonic epilepsy Etiology: AED aggravation Phenomenology: myoclonus (negative); status epilepticus (non convulsive) Localization: not applicable