Epileptic Disorders
MENUFixation-off sensitivity in epilepsies other than the idiopathic epilepsies of childhood with occipital paroxysms: a 12-year clinical-video EEG study Volume 11, issue 1, March 2009
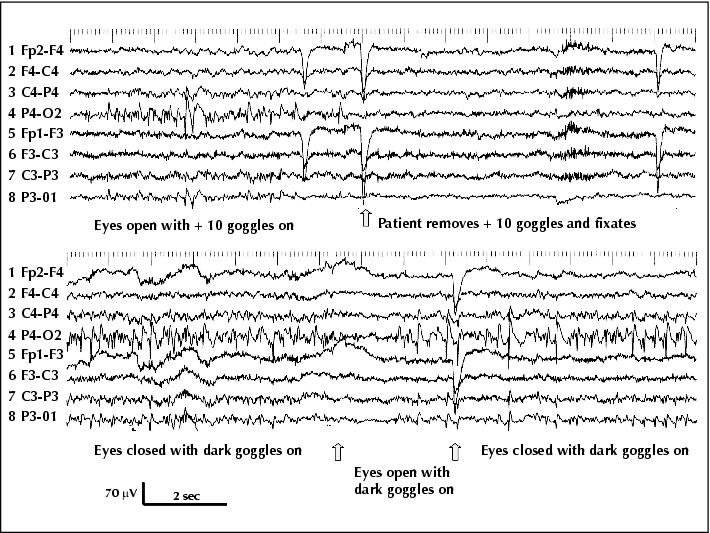
Figure 1 (Patient 1) Continuous right occipital spiking when eyes are open and fixation is impeded by + 10 translucent goggles (left half of upper trace); spiking immediately disappears upon visual fixation (right half of upper trace). Ongoing right occipital spiking when the patient wears dark goggles (lower trace): eye opening temporarily blocks spiking, but spikes resume after one second despite the fact that eyes remain open (middle part of lower trace, in-between the two arrows). Note the diffusion of the spikes to the left occipital areas (HFF 70 Hz, TC 0.3 s).

Figure 2 (Patient 1) Left: occipital spiking during stage II of sleep. Note that in contrast to the FOS related abnormalities in patients with idiopathic epilepsies that tend to attenuate during drowsiness along with the alpha rhythm dropout, occipital paroxysms here do not seem to have lost their vigour compared to wakefulness and they were also abundant during light drowsiness (HFF 70 Hz, TC 0.3 s). Right: brain MRI of patient 1. Coronal FLAIR (A) and axial TW2 sequences (B) show a sizable dysplastic area with cortical thickening, blurred grey-white matter junction and faint cortical and subcortical T2 high signal in the right lateral occipital gyri (arrows).
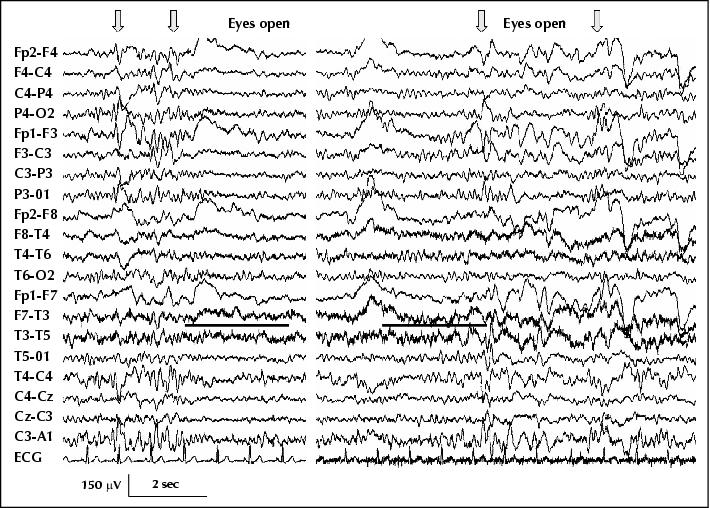
Figure 3 (Patient 3) Left trace: diffuse, bilateral bursts of high voltage sharp activity when eyes are closed (arrows), and polymorphic slow activity over the left temporal area that persists on eye opening (underlined). Bilateral sharp activity occurred only when eyes were shut, and was consistently inhibited by eye opening. Note also that eye opening completely blocks the alpha rhythm. Right trace: eye opening does not inhibit the bilateral bursts of sharp activity or the alpha rhythm when the patient wears semi-transparent goggles. Complete darkness (using opaque underwater goggles) also showed perseverance of the alpha rhythm on eye opening and diffuse bursts of sharp activity (HFF 70 Hz, TC 0.3 s).

Figure 4 (Patient 8) Top: âeyes closedâ-related posterior high voltage 3-4 Hz delta rhythm with intermixed spikes and more generalised bursts of spike-wave activity (white arrows). Both block on eye opening. Note also the brief discharge of polyspike-waves that occur on eye closure (grey arrow). Bottom: the patient wears completely opaque goggles (absolute darkness). Both posterior high voltage delta rhythm (black arrow) and the generalised bursts of sharp activity (white arrows) now occur when eyes are open. Note that in complete darkness, the eye closure paroxysms disappear (grey arrow) (compare with top trace and also with figure 5; semi-transparent goggles had similar effects confirming FOS (HFF 70 Hz, TC 0.3 s).

Figure 5 (Patient 8) Top left: typical eye-closure paroxysms (ECP) of diffuse polyspike and wave that were consistently associated with eyelid myoclonia and variably with brief impairment of cognition. Top right: generalised photoparoxysmal response at 18 Hz associated with eyelid myoclonus. Bottom: Spontaneous typical absence when eyes were open. The regular 3Hz spike-wave discharge starts with a bilateral burst of polyspike-wave and is associated with profound impairment of cognition, eyelid fluttering and minor automatisms. Similar long absences occurred with eyes closed, spontaneously or during hyperventilation, in which case they were preceded by a run of high voltage occipital rhythmic delta (OIRDA) (HFF 70 Hz, TC 0.3 s).

Figure 6 (Patient 12) Complex relationship between photosensitivity and FOS; the patient has been photosensitive since her first video EEG at the age of 14 years, and this video EEG at the age of 20 shows for the first time FOS that concerns her bilateral occipital needle-like spikes/polyspikes (right bottom trace, in which occipital spikes are associated with eye watering). The range of photosensitivity is from 30Hz (bottom left trace) to 12 Hz (generalized PPR of posterior onset in middle trace). Note that the latter is followed by FOS bilateral posterior spikes (their onset is marked by the grey arrow) that persist throughout the eyes-closed period. The possibility that the bilateral posterior spikes relate to eye closure and not to FOS is negated by the right bottom trace, in which there are no eye closure abnormalities and the posterior spiking starts one second after eye closure, also marked by a grey arrow. IPS at 10Hz (upper trace) or less appears to âsuppressâ the FOS posterior spiking, as this appears when IPS ceases and after a brief (0.5 sec) of electrical suppression (grey arrow) (HFF 70 Hz, TC 0.3 s).

Figure 7 (Patient 13) Bilateral posterior spike-wave bursts (white arrows) diffusing forwards (grey arrows) occur during eye-closed state (left and right parts of trace), and persist when eyes open but fixation is impeded using semi-transparent goggles (middle part of trace). Note also the persistence of the alpha rhythm throughout the âeyes openâ period. The EEG normalised every time she opened her eyes and fixated (HFF 70 Hz, TC 0.3 s).

Figure 8 (Patient 14) Top left trace: bilateral synchronous occipital paroxysms (OP) with eyes closed are promptly inhibited by eye opening and visual fixation. Top right trace: ongoing OP irrespective of the state of the eyes as long as dark goggles are on. Lower trace: eyes are open with dark goggles on (left third), dark goggles off and visual fixation (middle third), and + 10 translucent goggles on (right third). Sub-clinical generalised polyspike â wave discharges are marked with arrows (HFF 70 Hz, TC 0.3 s).

